Abstract
Background:
The association between cost-sharing and receipt of medication for opioid use disorder (MOUD) is unknown.
Methods:
We constructed a cohort of 10,513 commercially insured individuals with a new diagnosis of opioid use disorder (OUD) and information on insurance cost-sharing in a large national de-identified claims database. We examined four cost-sharing measures: 1) pharmacy deductible; 2) medical service deductible; 3) pharmacy medication co-pay; and 4) medical office co-pay. We measured MOUD (naltrexone, buprenorphine, or methadone) initiation (within 14 days of diagnosis), engagement (second receipt within 34 days of first), and 6-month retention (continuous receipt without 14-day gap). We used multivariable logistic regression to assess the association between cost-sharing and MOUD initiation, engagement, and retention. We calculated total out-of-pocket costs in the 30 days following MOUD initiation for each type of MOUD.
Results:
Of 10,513 individuals with incident OUD, 1,202 (11%) initiated MOUD, 742 (7%) engaged, and 253 (2%) were retained in MOUD at six months. A high ($1,000+) medical deductible was associated with a lower odds of initiation compared to no deductible (odds ratio: 0.85, 95% CI: 0.74-0.98). We found no significant associations between other cost-sharing measures for initiation, engagement, or retention. Median initial 30-day out-of-pocket costs ranged from $100 for methadone to $710 for extended-release naltrexone.
Conclusion:
Among insurance plan cost-sharing measures, only medical services deductible showed an association with decreased MOUD initiation. Policy and benefit design should consider ways to reduce cost barriers to initiation and retention in MOUD.
Keywords: opioid use disorder, medication treatment, insurance, initiation, retention
Introduction
The United States is facing a crisis of opioid-related harms, including opioid use disorder and overdose. In some states nearly 5% of Americans are living with opioid use disorder (OUD)1 and over the past decade the rate of fatal overdose has risen considerably, driven by the proliferation of synthetic opioids as the synthetic opioid-involved death rate increased 1,040% from 2013 to 2019.2 This increase in overdose deaths has prompted policy and practice efforts to increase access to treatment for OUD3 and implementation of overdose prevention programs. Despite these efforts, highly effective medications for OUD (MOUD) remain underutilized4 and discontinuation is common.5
In the United States, health insurance coverage is central to MOUD access. Insurance plans routinely cover MOUD, and expanding insurance encourages uptake: a 2018 evaluation of Medicaid expansion under the Affordable Care Act showed an 18% increase in OUD treatment compared to non-expansion states.6 While this evidence suggests that expansion of coverage benefits treatment generally, previous work has shown variation in initiation and retention among different types of commercial insurance5 and between commercial coverage and Medicare Advantage coverage.7 However, the source of this variation is unknown, particularly the impact of cost-sharing policies on treatment initiation and retention among commercially insured patients. Insurance benefit designs often include cost-sharing features, including deductibles and co-payments, to limit expenditures on more expensive treatments and reduce the perceived risk of a potential moral hazard observed when individuals consume more services when shielded from their costs.8 Insurers do not determine the choice of MOUD – which should come from a shared decision making process between a patient and provider9 – but MOUD can vary in cost,10 and there may be cost implications to a patient based on their insurance plan design. While work in other care contexts11,12 has examined the impact of cost-sharing, the impact of cost-sharing on MOUD access has not been comprehensively explored.
Understanding the impact of insurance cost-sharing designs on MOUD initiation and adherence is critical for increasing access to these effective, but underutilized5 MOUD. We sought to address this research gap by evaluating the association of cost-sharing features of insurance benefit design on initiation of, and retention and engagement to MOUDs among individuals with commercial insurance and newly diagnosed OUD in the United States.
Methods
Data
We used de-identified administrative claims data from the OptumLabs® Data Warehouse (OLDW), which includes medical and pharmacy claims, laboratory results, and enrollment records for commercial and Medicare Advantage enrollees. The database contains longitudinal health information on enrollees representing a diverse mixture of ages, ethnicities and geographical regions across the United States.13 We identified individuals with incident OUD between October 2015 and July 2019. We restricted the sample to individuals with commercial insurance as full cost-sharing benefit design information was not available for Medicare Advantage enrollees. OLDW data are statistically de-identified, and the Boston University Medical Center Institutional Review Board deemed this research exempt. Methods for study inclusion and data analysis were prespecified in a registered protocol on Open Science Framework (https://osf.io/wmz7j/) and included as a Supplemental Appendix.
Cohort definition
We identified a cohort of individuals aged 16 and older with OUD based on a previously defined algorithm (Supplemental Appendix).7 First, we included individuals with one or more inpatient claim or two or more outpatient claims with diagnosis codes for opioid dependence occurring within 90 days of each other and that did not occur during a long-term opioid prescribing episode. We excluded those with the diagnosis of dependence during a long-term prescribing episode, defined as three or more opioid dispensings at least 21 days apart and lasting at least 84 days, because individuals with physical dependence due to long-term prescribed opioid use may not have OUD.14 Second, we included individuals with one or more inpatient or outpatient claims with diagnosis codes for opioid dependence, use, or abuse with a confirmatory event within 90 days of the diagnosis. Confirmatory events included evidence of opioid overdose, evidence of MOUD, inpatient stay at a detoxification or rehabilitation facility, or diagnosis of an injection-related infection (Supplemental Appendix). The earliest data of recorded diagnosis or confirmatory event was the index date and marked an individual’s entry into the cohort. We required every individual to have 3 months of enrollment data prior to this index date (washout) to establish this was an incident OUD diagnosis. Following cohort creation, very few data elements had missing values. For the single variable with missing values, urbanicity, missingness was rare (~0.3% of observations) and we parameterized missing values as a separate missing category in all subsequent analyses. For comorbidities, medication treatments, and outcomes, there were no missing data because the variables were defined by the presence of diagnosis or procedure, or prescription fills. We interpreted the absence of such claims as the absence of the condition.
Exposure
We derived four key insurance cost-sharing design exposures from the enrollees benefit design information: pharmacy deductible, medical service deductible, medical office copay, and pharmacy medication copay. Deductibles are an amount an enrollee must spend before the insurance plan starts paying for covered services. For example, if a deductible is $1,000, an enrollee needs to pay 100% of utilization costs up to $1,000 out-of-pocket before insurance plan coverage begins. Following the deductible, insurance coverage varies by plan and by service, with some plans covering some services 100%, and others using a coinsurance mechanism where a portion of any costs after the deductible are covered by the insurance plan. Copays are fixed out-of-pocket expenses for certain covered services such as office visits or medications. Copays are typically charged after a deductible has been met, although they are sometimes charged before as well. We dichotomized pharmacy and medical deductibles as “high” ($1,000 or more) vs. “not high” reflecting prior literature.15 We defined medical and pharmacy copays as high or low, categorizing copays as high if they were in the top 50% of all copays in the cohort. For medical plans, we used the copay based on an office visit. For medications, the pharmacy copay depends on the tier of the drug, ranging from tier 1, generics favored by the insurer with the lowest pharmacy copay, to tier 4, which are often branded specialty drugs and have the highest copay. We did not have access to the formularies of each insurance plan (which describe each plan’s unique tier system) but did know the copayment levels for each tier for each plan. We used tier 2 as our pharmacy copay as we found examples of large plans categorizing generic MOUD as tier 2.16
Outcome measures
We assessed the care cascade of MOUD initiation, engagement, and retention among individuals with newly diagnosed OUD. MOUD was defined as receipt of buprenorphine or naltrexone, either oral naltrexone or extended-release injectable naltrexone (XR-NTX). We used either national drug code information from outpatient pharmacy claims or Current Procedural Terminology (CPT) or Healthcare Common Procedure Coding System (HCPCS) coding for in-person administration of injectable formulations from the medical claims (Supplemental Appendix). We identified receipt of methadone as MOUD using HCPCS H0020 for in-person administration through an opioid treatment program. Methadone receipt via pharmacy claims would be limited to treatment targeting pain and was not considered MOUD. Consistent with Washington Circle initiation, engagement, and retention performance measures,17 we defined MOUD initiation as receipt within 14 days of OUD diagnosis. Second, we defined engagement as receiving a second MOUD within 34 days of the first. Finally, we defined retention as receiving an MOUD consistently over 180 days, without a gap of more than 14 days between the end of one prescription fill or administration and the beginning of the next. The coverage period each prescription was defined based on the documented days supplied of a pharmacy-dispensed medication, defined as one day for daily-dosed methadone, or defined as 28 days for in-office administrations of injectable formulations.18,19 We also summarized out-of-pocket costs in the 30-days after MOUD initiation, as this period is critical for measuring costs, subsequent utilization, and the effectiveness of early treatment.20–22 These costs were the actual costs owed by patients reflecting total medical and pharmacy utilization in the 30-day period. We also calculated out-of-pocket costs for times matching our engagement (34 days) and retention (180 days) outcomes.
Other variables
We included additional demographic and clinical characteristics to describe the cohort and as potential confounders. Demographic characteristics included an individual’s age, sex, and urbanicity of residence, defined as metropolitan, micropolitan, small town, rural, or missing/unknown. We examined the quarter of the insurance coverage year at the index date to reflect non-OUD spending that may reduce the deductible burden through the year. For clinical characteristics, we measured whether an individual had a diagnosed non-OUD substance use condition, a mental health comorbidity, and assessed a modified Elixhauser score that excluded comorbidities examined separately such as mental health and substance use. We also included a measure of opioid-related overdose in the three months prior to the index date, as this can predict future treatment,4 as well as injection-related infection (including skin and soft-tissue infections and endocarditis, see Supplemental Appendix) and a diagnosis of Hepatitis C virus at any time to capture more severe use patterns.
Analysis
We characterized the MOUD care cascade by assessing the total number of individuals identified with incident OUD and the proportion reaching initiation, engagement, and retention. We summarized the demographic, cost-sharing, and clinical covariates of the full analytic cohort. To assess the association between cost-sharing and each outcome, we conducted a logistic regression predicting initiation, engagement, and retention as a function of our four cost-sharing measures and demographic and clinical control variables. We limited the sample for each regression model to participants reaching the prior stage. So, we modeled the odds of initiating conditional among those with incident OUD, the odds of engagement among those initiated, and retained among those engaged. Finally, we summarized the total out-of-pocket cost-sharing expenditures in the 30 days following initiation overall and by type of MOUD initiated, as well as stratified by cost-sharing design. We used an ANOVA test to examine the difference among MOUD type and a post-hoc Tukey Test to determine which MOUDs were driving the difference, if any.
Supplemental Analyses and robustness checks
We conducted several supplemental analyses to check the robustness of our results. First, we repeated these regression models stratified by the type of MOUD initiated to evaluate whether cost-sharing patterns differed by type of medication initiated. Second, we evaluated engagement and retention regression results using the full sample rather than restricting to those who had achieved the prior stage. This provided a population-level estimate for factors associated with engagement and retention generally, rather than restricting our focus to only those eligible for engagement or retention (e.g., those who had initiated and engaged, respectively). Third, we investigated the potential multicollinearity among our cost-sharing variables by estimating the Variance Inflation Factor (VIF). Further detail and results of these analyses are located in the Supplemental Appendix.
Results
We identified 10,513 individuals with incident OUD between October 2015 and July 2019 (Table 1). Most of our cohort was male (63%), between the ages of 25 and 64 (83%), and lived in a metropolitan region (86%). Concurrent alcohol use disorder and other concurrent non-opioid substance use were low (9% and 3% or less, respectively). Seventeen percent of the sample had diagnosed depression, and 21% diagnosed anxiety disorder. For cost-sharing, most individuals had a high medical deductible (63% compared to 37% with a medical deductible under $1,000) while pharmacy deductibles were less common (12% had a pharmacy deducible of $1,000 or more). The median cutoff value for copays was $35 for pharmacy copay and $30 for a medical office copay. Because cost-sharing values are not discrete values, 21% of individuals were identified as having a high pharmacy copay and 32% of individuals a high medical copay (Table 1).
Table 1:
Cohort characteristics of commercially insured patients 16 and older with OUD between 2015-2019
| Full cohort | ||
|---|---|---|
| n | % | |
| TOTAL | 10,513 | 100% |
| Initiating medication | ||
| Not initiated | 9,311 | 89% |
| Buprenorphine | 574 | 5% |
| Methadone | 149 | 1% |
| Oral naltrexone | 382 | 4% |
| Injectable naltrexone | 97 | 1% |
| Rx deductible | ||
| <$1000 | 9,273 | 88% |
| $1000+ | 1,240 | 12% |
| Medical deductible | ||
| <$1000 | 3,856 | 37% |
| $1000+ | 6,657 | 63% |
| Rx T2 copay | ||
| at or below median | 8,260 | 79% |
| Above median cost | 2,253 | 21% |
| Medical office copay | ||
| at or below median | 7,194 | 68% |
| Above median cost | 3,319 | 32% |
| Calendar quarter of plan year at initiation | ||
| 1 | 2,033 | 19% |
| 2 | 1,873 | 18% |
| 3 | 3,976 | 38% |
| 4 | 2,631 | 25% |
| Sex | ||
| Male | 6,614 | 63% |
| Female | 3,899 | 37% |
| Age | ||
| 16-24 | 1,625 | 15% |
| 25-64 | 8,697 | 83% |
| 65+ | 191 | 2% |
| Region | ||
| Metropolitan | 9,073 | 86% |
| Micropolitain | 890 | 8% |
| Small town | 369 | 4% |
| Rural | 148 | 1% |
| Missing | 33 | 0% |
| Clinical covariates | ||
| Concurrent substance use | ||
| Sedative | 320 | 3% |
| Cocaine | 264 | 3% |
| Cannabis | 244 | 2% |
| Amphetamine | 254 | 2% |
| Hallucinogen | 34 | 0% |
| Alcohol | 942 | 9% |
| Prior opioid overdose | ||
| Yes | 46 | 0% |
| No | 10,467 | 100% |
| Injection-related infection | ||
| Yes | 122 | 1% |
| No | 10,391 | 99% |
| Hepatitis C | ||
| Yes | 120 | 1% |
| No | 10,393 | 99% |
| Mental health | ||
| Depression | 1,807 | 17% |
| Anxiety | 2,156 | 21% |
| ADHD | 617 | 6% |
| PTSD | 275 | 3% |
| Bipolar | 475 | 5% |
| Psychoses | 139 | 1% |
| Modified Elixhauser* | ||
| 0 | 7,268 | 69% |
| 1 | 1,667 | 16% |
| 2 | 736 | 7% |
| 3+ | 842 | 8% |
Modified to exclude substance use and mental health, which are modeled separately
Figure 1a depicts the cascade of medication care. Of those identified with incident OUD, 1,202 (11%) initiated medication treatment within 14 days. Of those, 742 (7% of total, 62% of those who initiated) were engaged in care with a 2nd receipt of medication, and just 253 (2% of total and 34% of those engaged) were retained in care at six months. Figure 1b depicts the cascade of medication care beginning at initiation stratified by medication type and reveals heterogeneity by medication. Due to sample size suppression rules, we combined oral and injectable naltrexone for this exercise. Methadone had the highest engagement proportion (91% of those initiating), followed by buprenorphine (69% engaged of initiated), and naltrexone (44%). Agonist medications had a higher proportion retained at six months (32% and 30% of initiated for buprenorphine and methadone, respectively) compared to antagonists (5% for naltrexone).
Figure 1:
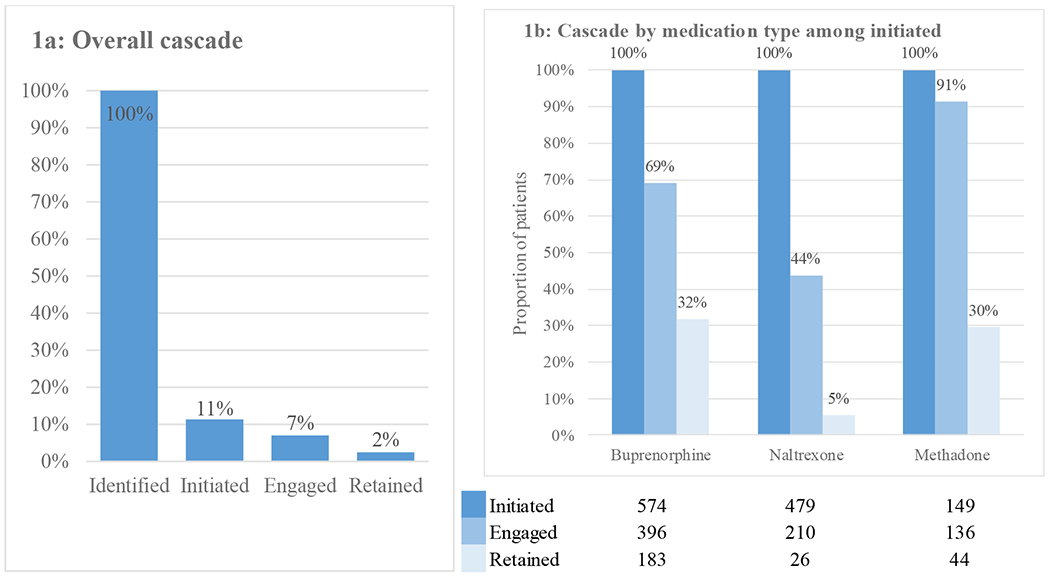
Initiation to, engagement in, and retention on medication treatment for opioid use disorder in a commercially insured population of 10,513 individuals diagnosed with opioid use disorder between 2015 and 2019
We detected an association of cost-sharing on MOUD initiation, but not engagement or retention. In the multivariable logistic regression predicting initiation after incident OUD, we found a high ($1,000+) medical deductible was associated with a lower odds of initiation (odds ratio [OR] = 0.85, 95% confidence interval [CI] 0.74-0.98) (Table 2). We found no significant association for pharmacy deductible, or either copay measure. No cost-sharing measures significantly predicted engagement or retention once initiated or engaged respectively. We found that those with a modified Elixhauser score greater than 0 had lower odds of initiating (OR ranging from 0.41-0.78 depending on the score), and female individuals were more likely to initiate (OR = 1.19, 95% CI 1.05-1.35). These findings were not evident when examining engagement or retention. We also found that being in the third quarter of the insurance plan coverage was associated with a higher odds of retention relative to the fourth quarter (OR = 1.85, 95% CI 1.26-2.70).
Table 2:
Multivariable logistic regression models predicting medication for opioid use disorder initiation, engagement, and retention.
| Initiation (of those with OUD, n=10,513) | Engagement (of those initiated, n=1,202) | Retention (of those engaged, n=742) | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Pharmacy deductible | |||
| <$1000 | Reference | Reference | Reference |
| $1000+ | 1.10 (0.91-1.33) | 0.99 (0.67-1.45) | 0.63 (0.38-1.05) |
| Medical deductible | |||
| <$1000 | Reference | Reference | Reference |
| $1000+ | 0.85 (0.74-0.98) | 1.11 (0.84-1.47) | 0.85 (0.61-1.19) |
| Pharmacy copay | |||
| Above median cost | 1.04 (0.90-1.21) | 1.33 (0.98-1.80) | 1.27 (0.89-1.81) |
| At or below median | Reference | Reference | Reference |
| Medical office copay | |||
| Above median cost | 1.02 (0.89-1.17) | 0.89 (0.68-1.18) | 0.91 (0.65-1.27) |
| At or below median | Reference | Reference | Reference |
| Current plan quarter | |||
| 1 | 1.11 (0.93-1.33) | 1.36 (0.93-2.01) | 0.63 (0.36-1.10) |
| 2 | 1.00 (0.83-1.22) | 0.74 (0.49-1.10) | 1.38 (0.87-2.19) |
| 3 | 0.91 (0.74-1.12) | 0.77 (0.51-1.18) | 1.85 (1.26-2.70) |
| 4 | Reference | Reference | Reference |
| Sex | |||
| Male | Reference | Reference | Reference |
| Female | 1.19 (1.05-1.35) | 1.05 (0.82-1.35) | 0.98 (0.72-1.33) |
| Age | |||
| 16-24 | 1.13 (0.59-2.16) | 0.61 (0.17-2.20) | 0.29 (0.06-1.35) |
| 25-64 | 1.71 (0.92-3.19) | 1.43 (0.42-4.90) | 0.76 (0.18-3.14) |
| 65+ | Reference | Reference | Reference |
| Region | |||
| Metropolitan | Reference | Reference | Reference |
| Micropolitain | 0.88 (0.70-1.12) | 1.04 (0.65-1.66) | 0.64 (0.34-1.21) |
| Small town | 0.83 (0.58-1.18) | 1.04 (0.50-2.18) | 1.71 (0.78-3.75) |
| Rural | 1.15 (0.71-1.86) | 1.18 (0.45-3.10) | 2.64 (1.00-6.97) |
| Missing | 1.57 (0.59-4.16) | 0.90 (0.15-5.57) | 0.75 (0.08-6.85) |
| Clinical covariates | |||
| Concurrent substance use | |||
| Sedative | 0.82 (0.56-1.19) | 0.50 (0.24-1.02) | 0.53 (0.14-195) |
| Cocaine | 0.92 (0.61-1.39) | 1.54 (0.69-3.46) | 4.49 (1.42-14.26) |
| Cannabis | 0.98 (0.64-1.50) | 2.10 (0.87-5.07) | 0.93 (0.19-4.54) |
| Amphetamine | 0.83 (0.54-1.28) | 0.87 (0.37-2.05) | 0.22 (0.02-2.08) |
| Hallucinogen | 1.16 (0.42-3.17) | 0.61 (0.08-4.51) | NC |
| Alcohol | 2.83 (2.33-3.44) | 0.79 (0.55-1.15) | 0.34 (0.18-0.63) |
| Prior opioid overdose | |||
| Yes | 1.03 (0.39-2.68) | 0.25 (0.04-1.65) | NC |
| No | Reference | Reference | Reference |
| Injection-related infection | |||
| Yes | 0.75 (0.37-1.50) | 1.99 (0.39-10.13) | 7.66 (1.69-34.69) |
| No | Reference | Reference | Reference |
| Hepatitis C | |||
| Yes | 1.63 (0.96-2.79) | 1.77 (0.54-5.75) | 1.56 (0.49-4.97) |
| No | Reference | Reference | Reference |
| Mental health | |||
| Depression | 0.91 (0.76-1.10) | 0.84 (0.58-1.23) | 0.59 (0.34-1.02) |
| Anxiety | 1.07 (0.91-1.27) | 0.92 (0.65-1.29) | 0.98 (0.62-1.54) |
| ADHD | 1.13 (0.88-1.45) | 1.03 (0.62-1.70) | 0.87 (0.44-1.72) |
| PTSD | 1.03 (0.70-1.51) | 1.61 (0.73-3.55) | 0.77 (0.22-2.73) |
| Bipolar | 0.87 (0.63-1.19) | 0.64 (0.34-1.20) | 0.68 (0.25-1.88) |
| Psychoses | 0.48 (0.24-0.97) | 1.56 (0.36-6.71) | NC |
| Modified Elixhauser score | |||
| 0 | Reference | Reference | Reference |
| 1 | 0.78 (0.66-0.93) | 1.10 (0.78-1.57) | 0.64 (0.40-1.03) |
| 2 | 0.58 (0.44-0.76) | 1.22 (0.68-2.17) | 1.20 (0.62-2.32) |
| 3+ | 0.41 (0.31-0.55) | 0.64 (0.35-1.16) | 0.62 (0.24-1.58) |
NC=not calculable due to small sample
Each model adjusts for all variables simultaneously and are multivariable models
We found that injectable naltrexone had the highest out-of-pocket spending 30 days after initiation, a median of $710, although there was significant variability and the interquartile range was large, ranging from $202 to $791 (Table 3). Buprenorphine, oral naltrexone, and methadone reported median 30-day expenditures of $160, $378, and $100, respectively, likely reflecting lower out-of-pocket costs associated with oral medications. While injectable buprenorphine is included in the total buprenorphine count, it represents a very small share of all buprenorphine. An ANOVA test confirms the 30-day out-of-pocket expenditures varied significantly by medication type (p<0.01). A post-hoc Tukey Test confirms the difference is largely driven by injectable naltrexone (the mean difference between injectable naltrexone and all other MOUD ranged from $346 to $1,099 with 95% confidence intervals that did not overlap with buprenorphine or methadone pairwise comparisons). The differences in out-of-pocket spending among the cost-sharing designs was less pronounced, with the larges difference in spending coming for those with a high pharmacy deductible (median $364, IQR $125-$1,124) compared to those with a pharmacy deductible under $1,000 (median $190, IQR $56-$636) (Table 3). Our findings were similar over longer time periods (34 and 180 days), with initiation of naltrexone associated with higher out-of-pocket spending over time (Supplemental Table 1)
Table 3:
Out-of-pocket costs 30 days after initiating medication for opioid use disorder, among those initiating medication treatment
| n | Median | IQR | |
|---|---|---|---|
| Among all initiations | 1202 | $210 | ($60-$682) |
| Medication type | |||
| Buprenorphine | 574 | $160 | ($48-$460) |
| Methadone | 149 | $100 | ($7-$317) |
| Oral NTX | 382 | $378 | ($101-$1152) |
| XR-NTX | 97 | $710 | ($202-$791) |
| Cost-sharing category | |||
| Pharmacy deductible | |||
| <$1000 | 1039 | $190 | ($56-$636) |
| $1000+ | 163 | $364 | ($125-$1124) |
| Medical deductible | |||
| <$1000 | 473 | $189 | ($56-$580) |
| $1000+ | 729 | $225 | ($66-$840) |
| Pharmacy copay | |||
| At or below median | 940 | $222 | ($70-$694) |
| Above median cost | 262 | $165 | ($46-$622) |
| Medical office copay | |||
| At or below median | 824 | $202 | ($60-$668) |
| Above median cost | 378 | $212 | ($66-$732) |
Results of supplemental analyses and robustness checks
First, in the analysis stratifying the regression by MOUD initiated, only XR-NTX initiations show a statistically significant association between high medical deductible and initiation (Supplemental Table 2). Other cost-sharing values were not significant for any other MOUD for either initiation or engagement, and numbers were too small to estimate MOUD-stratified models of retention at 180 days. Given the sample size, confidence intervals were wide in these sub-analyses. Second, our results were unchanged when we widened our sample in the engagement and retention analyses (Supplemental Table 3). Although changing the denominator increased the sample size of our engagement and retention analyses, we did not find an effect of any cost-sharing measure. Third, in our analysis of multicollinearity every variable in our model had a VIF<2 which indicates low multicollinearity.23
Discussion
In this analysis of more than 10,000 individuals with commercial insurance and a new diagnosis of OUD, only 11% initiated MOUD within 14 days and only 2% were retained on MOUD at 6 months. Of the pharmacy and medical cost-sharing designs we tested, a high medical deductible ($1,000 or more) was associated with a 15% reduction in the odds of initiating MOUD compared to a low deductible. We did not find cost-sharing to be a driving factor of subsequent MOUD receipt and longer-term retention.
While concerning, our findings of low rates of MOUD initiation and retention are consistent with past studies.5,7,24 These results add medical deductible to a substantial list of barriers to MOUD initiation that includes stigma, and accessibility of MOUD providers.25–27 We also attempted to capture temporal effects that may be related to out of pocket payment and insurance plan design. We had hypothesized that later quarters would have a higher odds of utilization relative to earlier quarters as individuals would have been more likely to both have met their deductible and, for some, reached their out of pocket maximum, reducing the out of pocket cost to access care. While the third quarter has statistically significantly higher odds than the first, as hypothesized, we are unsure why the third quarter would be higher than fourth, which was the assigned reference category. One possibility is confounding by calendar effects: while insurance plan coverage does not have to correspond to a calendar year, it often does, so the fourth quarter measure may be confounded with seasonal effects that may also pose barriers to care utilization and retention. Further work is needed to understand how the presence of a medical deductible may influence patient behavior, including the influence of out-of-pocket costs for urine drug testing, medical and behavioral health care visits, and monitoring.10,28 Notably, detoxification and inpatient treatment, common points of initial treatment seeking for individuals with OUD, are expensive and associated costs may deter seeking outpatient follow-up.29,30 While these services are important for some individuals, it is possible to initiate MOUD in an outpatient setting first. Delivery systems and benefit designs should be redesigned to promote low-threshold MOUD initiation and uptake.31
Our finding that cost-sharing was not associated with engagement or retention whereas the medical co-pay design was associated with lower initiation is an important insight for the field. The median total out-of-pocket costs in the first 30 days after initiating MOUD varied among medications, and 30-day costs of those initiating XR-NTX were at least double all others at a median of $710 while methadone ($100) and buprenorphine ($160) had the lowest median costs. The challenge to adherence is a well-known barrier to OUD care and its effectiveness,5,7,14,32,33 and establishing the key barriers to retention – cost and otherwise – is important for designing effective interventions. Indeed, our findings echo the retention challenges of the previous literature32 when we found just 11% of individuals with incident OUD initiated medication, 7% engaged, and just 2% were retained at 180 days. While our sample size decreases when assessing engagement and retention, and further research to confirm these findings is likely necessary, the lack of a clear signal of cost-sharing among those who have already initiated suggests barriers may change along the cascade. In other words, a copay may be a minor barrier relative to the major barriers that exist to receiving a diagnosis, linking to care, and finding a provider to prescribe medications. Our out-of-pocket findings by cost-sharing design support this insofar as the differences among cost-sharing plans are much less stark than for medications. The largest difference we found was in pharmacy deductible, where spending is expected to take place after initiating a medication. Rather than initiation, engagement, or retention, cost-sharing, particularly the medical deductible, may be a barrier to addiction care access in general, similar to a lack of physicians waivered to prescribe buprenorphine34,35 or stigma associated with OUD care, whereas it appears to not be a barrier to remaining engaged with medication treatment.36–38 More work is needed to identify the barriers to engagement and retention even when barriers to access have been successfully overcome.
Our findings are subject to several limitations. First, our cohort consisted of individuals with commercial insurance between 2015 and 2019, and our findings may not generalize to other populations. Commercially insured individuals may have differential response to cost-sharing than others including publically insured. While this research should be replicated in other populations, given that commercially insured patients also face low MOUD utilization and retention,5 it is important to understand barriers in this population. This study was a cohort study using data in a large claims database, and not a randomized trial designed to compare the different insurance cost-sharing approaches. Next, our limited sample size posed a challenge for predicting medication treatment cascade stratified by treatment type. Our finding that medical deductible is associated with initiation would seem to suggest that expensive office-based medications such as XR-NTX and newer formulations of injectable buprenorphine39 may face a particular barrier. However, we were unable to detect an association in our sample of 97 XR-NTX patients. While all medication treatments require costs beyond the medication itself (both to the patient in the form of out of pocket costs and to the insurer), future research determining how this barrier differs with medication treatment type would be an important consideration for patients and providers determining the best MOUD therapy. Opioid use disorder is a chronic, relapsing disease that, akin to other chronic conditions such as hypertension, benefits from long-term treatment40 and supportive measures such as rescue naloxone,41 so differences in costs will compound over time. Finally, commercial insurance coverage for methadone was not robust prior to 2017 and claims may not capture utilization of services for which members paid fully out-of-pocket for methadone treatment.
Conclusions
In a commercially insured population, we found that individuals with high medical deductibles had lower odds of initiating MOUD treatment compared to those with low deductibles. We did not find an association between cost-sharing and engagement or retention to treatment. Our findings suggest that cost-sharing may be a significant barrier to starting treatment, but more research is needed to understand the barriers to engaging in and remaining retained in care.
Supplementary Material
Acknowledgement:
This work was supported by the Robert Wood Johnson Foundation (grant 76358) and the National Institute on Drug Abuse (grant numbers R01DA046527 and P30DA040500).
Footnotes
All authors report no conflict of interest.
References
- 1.Barocas JA, White LF, Wang J, et al. Estimated Prevalence of Opioid Use Disorder in Massachusetts, 2011-2015: A Capture-Recapture Analysis. Am J Public Health 2018:e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL. Trends and Geographic Patterns in Drug and Synthetic Opioid Overdose Deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep 2021;70:202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkow ND, Wargo EM. Overdose Prevention Through Medical Treatment of Opioid Use Disorders. Ann Intern Med 2018;169:190–2. [DOI] [PubMed] [Google Scholar]
- 4.Larochelle MR, Bernson D, Land T, et al. Medication for Opioid Use Disorder After Nonfatal Opioid Overdose and Association With Mortality: A Cohort Study. Ann Intern Med 2018;169:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abuse Treat 2018;85:90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meinhofer A, Witman AE. The role of health insurance on treatment for opioid use disorders: Evidence from the Affordable Care Act Medicaid expansion. J Health Econ 2018;60:177–97. [DOI] [PubMed] [Google Scholar]
- 7.Wakeman SE, Larochelle MR, Ameli O, et al. Comparative Effectiveness of Different Treatment Pathways for Opioid Use Disorder. JAMA Netw Open 2020;3:e1920622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delbanco S, Murray R, Berenson R, Upandhyay D. Payment methods and benefit designs: how they work and how they work togeher to improve healthcare: Urban Institute; 2016. [Google Scholar]
- 9.Saunders EC, Moore SK, Walsh O, et al. Perceptions and preferences for long-acting injectable and implantable medications in comparison to short-acting medications for opioid use disorders. J Subst Abuse Treat 2020;111:54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy SM, McCollister KE, Leff JA, et al. Cost-Effectiveness of Buprenorphine-Naloxone Versus Extended-Release Naltrexone to Prevent Opioid Relapse. Ann Intern Med 2019;170:90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kay ES, Pinto RM. Is Insurance a Barrier to HIV Preexposure Prophylaxis? Clarifying the Issue. Am J Public Health 2020;110:61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciemins EL. The effect of parity-induced copayment reductions on adolescent utilization of substance use services. J Stud Alcohol 2004;65:731–5. [DOI] [PubMed] [Google Scholar]
- 13.OptumLabs. OptumLabs and OptumLabs Data Warehouse (OLDW) Descriptions and Citation. Eden Prairie, MN: n.p., July 2020. PDF. Reproduced with permission from OptumLabs. [Google Scholar]
- 14.Larochelle MR, Liebschutz JM, Zhang F, Ross-Degnan D, Wharam JF. Opioid Prescribing After Nonfatal Overdose and Association With Repeated Overdose: A Cohort Study. Ann Intern Med 2016;164:1–9. [DOI] [PubMed] [Google Scholar]
- 15.Galbraith AA, Ross-Degnan D, Soumerai SB, Rosenthal MB, Gay C, Lieu TA. Nearly half of families in high-deductible health plans whose members have chronic conditions face substantial financial burden. Health Aff (Millwood) 2011;30:322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.2020 complete drug list (formulary). Formulary ID Number 00020047, Version 9 Y0066_190628_124536_C v15.01. 2019. (Accessed 2 December, 2020, at https://www.aarpmedicareplans.com/alphadms/ovdms10g/groups/ov/@ov/@highrespdf/documents/highrespdf/4624550.pdf.)
- 17.Garnick DW, Lee MT, Chalk M, et al. Establishing the feasibility of performance measures for alcohol and other drugs. J Subst Abuse Treat 2002;23:375–85. [DOI] [PubMed] [Google Scholar]
- 18.Medication Guide: Vivitrol (naltrexone for extended-release injectable suspension). 2013. at https://www.fda.gov/downloads/Drugs/DrugSafety/UCM206669.pdf.)
- 19.Sublocade Package Insert. 2017. at https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209819s000lbl.pdf.)
- 20.Busch SH, Fiellin DA, Chawarski MC, et al. Cost-effectiveness of emergency department-initiated treatment for opioid dependence. Addiction 2017;112:2002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shafi S, Collinsworth AW, Copeland LA, et al. Association of Opioid-Related Adverse Drug Events With Clinical and Cost Outcomes Among Surgical Patients in a Large Integrated Health Care Delivery System. JAMA Surg 2018;153:757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts AW, Saloner B, Dusetzina SB. Buprenorphine Use and Spending for Opioid Use Disorder Treatment: Trends From 2003 to 2015. Psychiatr Serv 2018;69:832–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’brien RM. A Caution Regarding Rules of Thumb for Variance Inflation Factors. Quality & Quantity 2007;41:673–90. [Google Scholar]
- 24.Olfson M, Zhang VS, Schoenbaum M, King M. Trends in Buprenorphine Treatment in the United States, 2009–2018. Jama 2020;323:276–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haffajee RL, Lin LA, Bohnert ASB, Goldstick JE. Characteristics of US Counties With High Opioid Overdose Mortality and Low Capacity to Deliver Medications for Opioid Use Disorder. JAMA Netw Open 2019;2:e196373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joudrey PJ, Chadi N, Roy P, et al. Pharmacy-based methadone dispensing and drive time to methadone treatment in five states within the United States: A cross-sectional study. Drug Alcohol Depend 2020;211:107968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakeman SE, Rich JD. Barriers to Medications for Addiction Treatment: How Stigma Kills. Subst Use Misuse 2018;53:330–3. [DOI] [PubMed] [Google Scholar]
- 28.Jones ES, Moore BA, Sindelar JL, O’Connor PG, Schottenfeld RS, Fiellin DA. Cost analysis of clinic and office-based treatment of opioid dependence: results with methadone and buprenorphine in clinically stable patients. Drug Alcohol Depend 2009;99:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larochelle MR, Wakeman SE, Ameli O, et al. Relative Cost Differences of Initial Treatment Strategies for Newly Diagnosed Opioid Use Disorder. Medical Care 2020;Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan JR, Barocas JA, Murphy SM, et al. Comparison of Rates of Overdose and Hospitalization After Initiation of Medication for Opioid Use Disorder in the Inpatient vs Outpatient Setting. JAMA Netw Open 2020;3:e2029676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snow RL, Simon RE, Jack HE, Oller D, Kehoe L, Wakeman SE. Patient experiences with a transitional, low-threshold clinic for the treatment of substance use disorder: A qualitative study of a bridge clinic. J Subst Abuse Treat 2019;107:1–7. [DOI] [PubMed] [Google Scholar]
- 32.Morgan JR, Wang J, Barocas JA, et al. Opioid overdose and inpatient care for substance use disorder care in Massachusetts. Journal of Substance Abuse Treatment 2020;112:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan J, Schackman B, Weinstein Z, Walley A, Linas B. Overdose following initiation of naltrexone and buprenorphine medication treatment for opioid use disorder in a United States commercially insured cohort. Drug and Alcohol Dependence 2019;200:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenblatt RA, Andrilla CH, Catlin M, Larson EH. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann Fam Med 2015;13:23–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knudsen HK. The Supply of Physicians Waivered to Prescribe Buprenorphine for Opioid Use Disorders in the United States: A State-Level Analysis. Journal of Studies on Alcohol and Drugs 2015;76:644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hadland SE, Park TW, Bagley SM. Stigma associated with medication treatment for young adults with opioid use disorder: a case series. Addict Sci Clin Pract 2018;13:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bozinoff N, Anderson BJ, Bailey GL, Stein MD. Correlates of Stigma Severity Among Persons Seeking Opioid Detoxification. Journal of addiction medicine 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsen Y, Sharfstein JM. Confronting the stigma of opioid use disorder--and its treatment. Jama 2014;311:1393–4. [DOI] [PubMed] [Google Scholar]
- 39.Morgan JR, Walley AY, Murphy SM, et al. Characterizing initiation, use, and discontinuation of extended-release buprenorphine in a nationally representative United States commercially insured cohort. Drug Alcohol Depend 2021;225:108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. The Lancet 2019. [DOI] [PubMed] [Google Scholar]
- 41.Murphy SM, Morgan JR, Jeng PJ, Schackman BR. Will converting naloxone to over-the-counter status increase pharmacy sales? Health Serv Res 2019;54:764–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


