Abstract
Context
Genes causing familial forms of diabetes mellitus are only partially known.
Objective
We set out to identify the genetic cause of hyperglycemia in multigenerational families with an apparent autosomal dominant form of adult-onset diabetes not due to mutations in known monogenic diabetes genes.
Methods
Existing whole-exome sequencing (WES) data were used to identify exonic variants segregating with diabetes in 60 families from the United States and Italy. Functional studies were carried out in vitro (transduced MIN6-K8 cells) and in vivo (Caenorhabditis elegans) to assess the diabetogenic potential of 2 variants in the malate dehydrogenase 2 (MDH2) gene linked with hyperglycemia in 2 of the families.
Results
A very rare mutation (p.Arg52Cys) in MDH2 strongly segregated with hyperglycemia in 1 family from the United States. An infrequent MDH2 missense variant (p.Val160Met) also showed disease cosegregation in a family from Italy, although with reduced penetrance. In silico, both Arg52Cys and Val160Met were shown to affect MDH2 protein structure and function. In transfected HepG2 cells, both variants significantly increased MDH2 enzymatic activity, thereby decreasing the NAD+/NADH ratio—a change known to affect insulin signaling and secretion. Stable expression of human wild-type MDH2 in MIN6-K8 cell lines enhanced glucose- and GLP-1-stimulated insulin secretion. This effect was blunted by the Cys52 or Met160 substitutions. Nematodes carrying equivalent changes at the orthologous positions of the mdh-2 gene showed impaired glucose-stimulated insulin secretion.
Conclusion
Our findings suggest a central role of MDH2 in human glucose homeostasis and indicate that gain of function variants in this gene may be involved in the etiology of familial forms of diabetes.
Keywords: autosomal dominant diabetes, monogenic diabetes, gene mutation, Krebs cycle, glucose homeostasis, insulin secretion
Diabetes mellitus is a challenging global health problem, affecting more than 450 million people worldwide (1). While the vast majority of diabetic patients have polygenic type 1 diabetes (T1D) or type 2 diabetes (T2D), a small proportion of patients have monogenic forms of diabetes caused by single genetic defects (2). Among these latter forms, some are well recognized, such as the extremely rare neonatal diabetes (ND). Another example is the more common maturity onset diabetes of the young (MODY), which is inherited in an autosomal dominant manner and mostly occurs in nonobese young individuals, and for which several causative genes have been thus far identified (3).
Recently, we reported that about 3% of adult patients routinely diagnosed as having T2D are, in fact, members of families with multigenerational autosomal dominant diabetes not due to mutations in known monogenic diabetes genes (4, 5). We have shown that the clinical features of such patients are somehow intermediate between those of adult MODY patients and those of patients with typical T2D (4). Dissecting the genetic architecture of diabetes in these families is likely to provide insights into new mechanisms and pathways controlling glucose homeostasis and is, therefore, of utmost importance. To this purpose, we have been investigating 60 families (52 from the United States and 8 from Italy) with multigenerational diabetes and an apparent autosomal dominant inheritance, in which mutations in the 6 most common MODY genes (US families) (6) or in any of the known monogenic diabetes genes (Italian families) (5) had been previously excluded. Whole-exome sequencing (WES) had previously led to the identification of loss of function mutations in the APPL1 and SLC19A2 genes segregating with hyperglycemia in 3 of these families (6, 7).
In this study, we report 2 heterozygous pathogenic gain of function variants in the MDH2 gene that segregated with diabetes in a dominant fashion in 2 of the 60 multigenerational families described above. We further show that these 2 variants affect glucose-stimulated insulin secretion in MIN6-K8 mouse insulinoma cell lines and provide suggestive evidence that the same sequence changes at the orthologous mdh-2 affect glucose-induced insulin secretion in genetically modified C. elegans animal models.
Materials and Methods
Patients and Clinical Evaluation
Family studies
This study concerned 60 families with a pattern of occurrence of T2D consistent with an autosomal dominant mode of inheritance unlinked to mutations in known MODY genes who were recruited at the Joslin Diabetes Center in Boston, MA (N = 52) (8) and at the Scientific Institute Casa Sollievo della Sofferenza in San Giovanni Rotondo, Italy (N = 8) (4).
Study protocols and informed consent procedures were approved by the local institutional ethics committees in the United States and in Italy, as appropriate; all participants gave written consent. The study was carried out in accordance with the Declaration of Helsinki, as revised in 2000. Family members were classified as having diabetes, prediabetes, or normal glucose tolerance based on the ADA 2014 criteria (9).
Case–control study
In total, 1144 patients with early-onset diabetes recruited for suspicion of monogenic diabetes (age at diagnosis <40 years, HbA1c ≥6.5% or fasting plasma glucose ≥7 mmol/L or drug therapy for hyperglycemia, no islet cell autoantibodies) and 1376 controls without diabetes (HbA1c <6.5% or fasting plasma glucose <7 mmol/L, no drug therapy for hyperglycemia) were collected at Institut Pasteur de Lille, Lille University Hospital, Lille, France and gave written consent for genetic testing.
Genetic Analyses
For studies in the US and Italian families, WES and the filtering/prioritization pipeline used to reduce the number of variants for follow-up assessment were as previously reported (6). In the present study, we re-analyzed those same exome sequencing data according to the same bioinformatic pipeline but avoiding any gene prioritization by Genedistiller in the final step. Variants of interest were validated and analyzed for segregation with diabetes in the families using Sanger sequencing. Amplicons obtained from genomic DNA samples by polymerase chain reaction using gene-specific oligonucleotide primer pairs (available upon request) were subjected to direct sequencing in both forward and reverse directions on an automated AB 3130XL (Applied Biosystems, Foster City, CA) using the ABI PrismBigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). Results were analyzed with GeneScreen software (10). Variants were considered to segregate with diabetes if ≥80% of carriers >40 years old had developed diabetes, and there were no phenocopies whose phenotype was not consistent with either T1D or T2D.
In French patients, WES was performed according to the manufacturers’ protocol using Agilent (SureSelect Human All Exon), Twist (Human Core Exome), NimbleGen (MedExome), or Roche (HyperExome). MDH2 was accurately covered in each protocol.
Libraries were sequenced on Illumina systems (NovaSeq6000, HiSeq4000 or HiSeq2500), using a paired-end mode. A mean depth of coverage of at least 80× was obtained for each individual.
The analysis was focused on MDH2 variants that were very rare (ie, having a minor allele frequency [MAF] below 0.001 in GnomAD), were covered with more than 30 reads, and had a QUAL score higher than 200.
Studies in Human Islets
Islet isolation
Pancreata from 7 brain-dead nondiabetic (14.2% females, 76.7 ± 6.3 years, body mass index [BMI] = 27.0 ± 0.9 kg/m2) and 5 diabetic (40% females, 75.2 ± 4.5 years, BMI = 27.8 ± 1.3 kg/m2) donors were investigated in a case–control study, and pancreata from 18 additional nondiabetic brain-dead multiorgan donors (44.4% females, age = 58.7 ± 17.1 years, BMI = 25.8 ± 4.8 kg/m2) were investigated in correlative analyses. All samples were handled as previously reported (11) after informed consent was obtained in writing from family members. The islet isolation center providing the material for this study had permission to prepare isolated islets and use them for scientific research if they were not suitable for clinical islet transplantation, in agreement with national laws and institutional ethics rules (Comitato Etico per la Sperimentazione dell’Azienda Ospedaliera Universitaria di Pisa). Pancreatic islets were prepared by collagenase digestion and density gradient purification and cultured in M-199 culture medium, as previously reported (12).
MDH2 expression
Prime Time Standard qPCR Assays (Integrated DNA Technologies, Coralville, Iowa) were used to quantify relative gene expression levels of MDH2, GAPDH, β actin, and 18S on ABI-PRISM 7500 (Applera Life Technologies, Carlsbad, CA). Expression levels of MDH2 were calculated by using the comparative ΔCT method (13). MDH2 amounts were normalized for the geometric mean of GAPDH, β actin, and 18S and expressed as fold change.
Insulin secretion
Insulin secretion was determined as previously detailed (12). Briefly, following a 45-minute preincubation period at 3.3 mM glucose, batches of 15 islets of comparable size were kept at 37°C for 45 minutes in Krebs–Ringer bicarbonate (KRB) solution and 0.5% albumin, pH 7.4, containing 3.3 mM glucose. At the end of this period, the medium was completely removed and replaced with KRB containing 16.7 mM glucose and 3.3 mM glucose. After an additional 45 minutes of incubation, the medium was removed and stored at −20°C until insulin concentrations were measured by immunoradiometric assay (Pantec Forniture Biomediche). Data are expressed as stimulation index calculated by dividing stimulated insulin release at glucose 16.7 mM over basal insulin release (at glucose 3.3 mM).
In Silico Studies of MDH2 Variants and Molecular Dynamics Simulations
The possible deleterious effects of MDH2 Arg52Cys and Val160Met substitutions were evaluated by means of a previously reported scoring algorithm based on several in silico prediction tools (5). Atomic coordinates of human MDH2, in complex with a molecule of D-malate, were obtained from the Protein Data Bank (PDB id: 2dfd) and used for molecular dynamics (MD) simulations as described (14). In order to model both MDH252Cys and MDH2160Met, the wild-type MDH2 model was mutated in silico through UCSF Chimera (15). All 3 models were embedded in boxes, extending up to 12 Å, and solvated using the TIP3P water model. The tleap tool was used to add counter ions and thus neutralize the overall charge of the models. Each model was first energy minimized and then equilibrated for approximately 5 ns, in 1-fs time steps. MD simulations were performed 3 times on the 3 equilibrated models for 200 ns in 2-fs time steps, corresponding to a total of 100 million steps. A 10-Å cutoff was used for nonbonded short-range interactions; long-range electrostatics were treated with the particle-mesh Ewald method (16).
Temperature and pressure were maintained at 300 K and 101.3 kPa, respectively, using the Langevin dynamics and piston method (17). The Gromacs tools g_rms, g_rmsf, g_hbond, do_dssp, and g_mmpbsa (18) were used to calculate (1) root mean square deviation (RMSD), which measures the average distance between all heavy atoms (in this case Cα atomic coordinates) with respect to the X-ray structure; (2) per-residue root mean square fluctuation, measuring the deviation over time between the positions of the Cα atomic coordinates of each residue with respect to the X-ray structure; (3) hydrogen bonds; and (4) the dynamic cross-correlation maps (DCCMs), which allow one to investigate the long-range interactions of atoms and their correlated motions. Peaks, corresponding to the Cij elements of the map, are indicative of strong to moderate positive correlation (red to green), or of strong to moderate anticorrelation (dark to light blue) between residues i and j. Each of these was calculated for both wild-type and mutant proteins.
Enzyme Activity and NAD+/NADH Ratio Measurements in HepG2 Cells
Cloning and western blot analysis
A pLX304_MDH2 V5-tagged cDNA clone was obtained from the PlasmID Repository service of the DNA Resource Core at Harvard Medical School (Boston, MA). MDH2 cDNAs carrying the c.154C>T (p.Arg52Cys) or c.478G>A (p.Val160Met) substitutions were generated by means of the Quickchange Site-Directed Mutagenesis kit (Agilent), according to manufacturer’s instructions. HepG2 (Human Caucasian hepatocellular carcinoma) cells were obtained from ECACC (Salisbury, UK) and maintained at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s medium/F12 containing 10% fetal bovine serum (EuroClone S.p.A., Milano, Italy). Before experiments, HepG2 cells were seeded in 6-well plates and grown in Dulbecco’s modified Eagle’s medium/F12 complete medium for 48 hours. Cells were transiently transfected with either MDH2WT or MDH252Cys or MDH2160Met cDNA using TransIT-LT1 Transfection Reagents (Mirus Bio, Madison, WI) according to the manufacturer’s instructions. Anti-MDH2 (#18185, Abcam, RRID:AB_2893443; http://antibodyregistry.org/AB_2893443) and antirabbit (#sc-2004, Santa Cruz, RRID:AB_631746; http://antibodyregistry.org/AB_631746) were used as primary and secondary antibodies for the detection of MDH2 protein. Anti-beta-actin (#sc-47778, Santa Cruz, RRID:AB_631736; http://antibodyregistry.org/AB_631736) and antimouse (#sc-2005, Santa Cruz, RRID:AB_631736) were used as primary and secondary antibodies for the detection of beta-actin protein. Western blotting was performed as previously described (19).
MDH2 enzymatic activity and NAD+/NADH ratio measurements
MDH2 enzymatic activity and NAD+/NADH ratio were evaluated by means of the Mitochondrial Malate Dehydrogenase (MDH2) Activity Assay Kit (#ab119693, Abcam) according to the manufacturer’s instructions. MDH2 enzymatic activity was analyzed by longitudinal linear models after subtracting untransfected HepG2 cells data from those obtained in HepG2_MDH2WT, and HepG2_MDH252Cys cells and computed over 4 independent experiments for each experimental condition.
The NAD+/NADH ratio was evaluated by NAD+/NADH Quantification Kit (#MAK037, SIGMA). Briefly, after washing with cold phosphate-buffered saline, 3 × 105 cells were sonicated in 400 µL of extraction buffer and centrifuged at 13 000g for 10 minutes. The extracted supernatant was deproteinized by filtering through a 10 kDa cut-off spin filter and the NAD+/NADH ratio quantified at 450 nm, according to manufacturer’s instruction. Differences in NAD+/NADH ratio between HepG2_MDH2WT and HepG2_MDH252Cys were evaluated by Student’s t test.
Glucose-stimulated Insulin Secretion in Stably Transfected MIN6-K8 Cells
Cell culture
The mouse insulinoma cell line MIN6-K8 was kindly provided by Prof. J. Miyazaki, Osaka University (20). Cells were maintained in Dulbecco’s Modified Eagle’s Medium (#D5648, Sigma) containing 4 mM L-glutamine, and 25 mM glucose, supplemented with 10% fetal bovine serum (HyClone), 50 µM beta-mercaptoethanol (#M3148, Sigma), and 0.1 mg/mL streptomycin, 100 U/mL penicillin (#30-002-CI, Corning), at 37°C with 5% CO2 humidified condition.
Lentiviral construction and transduction
Lentiviral particles for delivering MDH2WT, MDH252Cys, and MDH2160Met were generated following the protocol from the TRC Library Database, Broad Institute. A 3-plasmid combination (9 μg of pLX304, 9 μg of dR8.91, and 0.9 μg of VSV-G) was cotransfected into 3.8 × 106 293T cells seeded on a 10-cm dish by using Lipofectamine™ 2000 (#11668027, Invitrogen). The supernatant was collected at 24 and 48 hours after transfection. After filtration, viral particles in supernatants were concentrated by using ultracentrifugation and transduced into MIN6-K8 cells. Transduced cells underwent selection for 14 days in the presence of 3 µg/mL Blasticidin (# A1113903, Gibco).
Determination of glucose-stimulated insulin release
MIN6-K8 cells stably expressing either MDH2WT, MDH252Cys, or MDH2160Met at 80% confluence were washed twice with KRB buffer (129 mmol/L NaCl, 4.8 mmol/L KCl, 2.5 mmol/L CaCl2, 1.2 mmol/L MgSO4, 1.2 mmol/L KH2PO4, 5 mmol/L NaHCO3, 10 mmol/L Hepes, and 0.1% bovine serum albumin) and preincubated for 2 hours in KRB buffer with 2.8 mM glucose. After discarding the pre-incubation buffer, cells were incubated in KRB buffer with 2.8 mM glucose for 1 hour, followed by KRB buffer with 16.7 mM glucose, with or without 50 nM GLP1 (#G3265, Sigma) for 1 hour. Insulin levels in supernatant were determined by means of the ultrasensitive mouse insulin ELISA kit (#90080, Crystal Chem, RRID:AB_2783626; http://antibodyregistry.org/AB_2783626) and adjusted for milligram protein of cell lysate.
Western blotting analysis
Cells cultured under standard conditions were lysed in RIPA buffer (Thermo Scientific) with protease inhibitor cocktail (#4693159001, Roche). Protein concentrations were determined by bicinchoninic acid protein assays (#89901, Thermo Scientific). Twenty micrograms of protein were separated onto sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane (#10600002, GE Healthcare). Immunostaining was performed using rabbit anti-MDH2 (1:10 000, #ab181873, Abcam, RRID:AB_2893443) and rabbit anti-α/β tubulin (1:1000, #2148, Cell Signaling, RRID:AB_2288042; http://antibodyregistry.org/AB_2288042) as primary antibodies and polyclonal swine antirabbit immunoglobulins conjugated with horseradish peroxidase (1:1000, #P0217, Agilent, RRID:AB_2728719; http://antibodyregistry.org/AB_2728719) as secondary antibody. Immunostaining signal was visualized using the ECL™ Prime Western Blotting System (#RPN2232, GE Healthcare). Band intensities were analyzed by densitometry using ImageJ software.
Caenorhabditis elegans Studies
Strains and culture
In order to explore the functional impact of the human MDH2 mutation on insulin secretion in vivo, we used the nematode Caenorhabditis elegans as an experimental model given that (1) this organism has a gene (mdh-2) orthologous to human MDH2; (2) insulin signaling is highly conserved between nematodes and humans (21); (3) C. elegans has already been successfully used to model T2D and other metabolic diseases (22, 23). The Bristol N2 and the GR1455 [mgIs40(daf-28p::GFP)] strains were obtained from the Caenorhabditis Genetics Center (University of Minnesota, MN). Culture, maintenance, and genetic crosses were as described (24).
Lines carrying the mdh-2 His56Cys and Val164Met alleles (namely cer10[H56C] and cer18[V164M], homologs of the human Arg52Cys and Val160Met alleles, respectively) were generated by CRISPR-Cas9 genome editing, as previously reported (25). Briefly, 20 wild-type animals were injected with a mix containing 500 ng/μL Cas9 (Labomics S.A.), 350 ng/μL ALT-R CRISPR tracrRNA (IDT), 170 ng/μL dpy-10 crRNA, 25 ng/μL ssODN dpy-10, 350 ng/μL mdh-2 crRNA (5′-TGACGACGTCGTAGAGGGCGAGG-3′), and 100 ng/μL of the mdh-2(cer10[H56C])-ssODN (5′-GAATTGGACAGCCATTGGGTCTCCTTCTCAAGCAGGATCCACTTGTTGCTTGTCTGGCACTGTATGATGTCGTCAATACTCCAGGAGTTGCCGCCGATCTTTCGCACA-3′) or the mdh-2(cer18[V164M])-ssODN (5′-TCAATTCTACTGTCCCAATCGCTTCCGAGGTCCTTAAAAAAGCCGGAATGTACGATCCAAAACGTGTCTTCGGAGTGACA-3′). Worms were then recovered on normal growth medium (NGM) plates at 20°C. Animals with a roller (rol) or a dumpy (dpy) phenotype were isolated, as well as pools of 5 wild-type worms from those plates. To isolate mutant animals, polymerase chain reaction amplification was performed using a single forward primer (5′-TCTCTTCCAGCAAAGACCCT-3′ mdh-2(cer10[H56C]) or 5′-CCGGAGTTCCACGTAAACCA-3′ mdh-2(cer18[V164M])) and 2 reverse primers annealing specifically with the mutated (5′-GACATCATACAGTGCCAGACAAG-3′ mdh-2(cer10[H56C]) or 5′-TTGGATC GTACATTCCGGCT-3′ mdh-2(cer18[V164M])) or the wild-type sequence (5′-AACGATTCCTGCGTTGGT-3′ mdh-2(cer10[H56C]) or 5′-TTGGGTCATAGACACCAGCC-3′ mdh-2(cer18[V164M])).
Two strains carrying the His56Cys substitution were generated: CER206 mdh-2(cer10)III and CER208 mdh-2(cer10)III. Two strains carrying the Val164Met substitution were also generated: CER230 mdh-2(cer18)III and CER231 mdh-2(cer18)III. All strains were out-crossed twice to remove possible off-target mutations. Strains sharing the same genotype exhibited an equivalent phenotype.
Assessment of “bagging”
Nematodes were cultured at 20°C. At the L4 stage, animals were transferred to agar plates containing NGM with 112 mM D-glucose added. Adults were moved to fresh NGM plates every 2 days to prevent progeny contamination. Occurrence of egg retention in the uterus and internal hatching (Bag-of-worms phenotype) was scored for 10 days using a Leica MZ10F dissecting microscope.
DAF-28/insulin expression/secretion
mdh-2(cer10[H56C]) and mdh-2(cer18[V164M]) mutant nematodes were crossed with animals expressing green fluorescent protein (GFP) under control of the daf-28 promoter (mgIs40(daf-28p::GFP)) as previously reported (24, 26). After each cross, the genotype was confirmed by direct sequencing of the appropriate genomic region. GFP expression was scored in adult animals belonging to 2 different clones and grown at 20°C with or without 30 mM D-glucose using a Nikon Eclipse 80i instrument equipped with differential interference contrast. Mean fluorescence intensity was measured by using the NIH ImageJ software.
Statistical Analyses
Data as obtained from at least 3 independent experiments performed in duplicate were expressed as means ± SD and analyzed using the paired Student t test, Fisher exact test, or longitudinal linear models, as appropriate. Survival curves were assessed by Kaplan–Meier analysis. Correlations were evaluated by Pearson’s correlation. All statistical analyses were performed using SAS Software, Release 9.4 (SAS Institute, Cary, NC, USA). Two-sided P values < .05 were considered to be statistically significant.
Results
WES and Sequence Analysis
By re-analyzing existing WES data from 52 families from the United States (6), employing a bioinformatic approach that did not prioritize genes based on their functions, we identified 972 possibly deleterious variants that were very rare (MAF <0.00001) or absent from public databases. Of these, 135 variants in 126 genes showed evidence of segregation with hyperglycemia in these families (data not shown). No pathogenic or likely pathogenic variants, as classified according to American College of Medical Genetics and Genomics and the Association for Molecular Pathology guidelines for variant interpretation (27), were found in established MODY genes that had not been included in the original screening.
Focusing on variants in genes with a possible involvement in glucose metabolism, we identified a missense variant (c.154C>T; p.Arg52Cys) in the malate dehydrogenase 2 gene (MDH2, NM_005918.2) showing strong evidence of segregation with hyperglycemia in 1 of the families (Fig. 1A). This is a very rare variant, observed in gnomAD v3.1.1. (http://gnomad.broadinstitute.org) in 1 out of 152 116 chromosomes and not observed in non-Finnish Europeans.
Figure 1.
MDH2 variants identified in 2 study families and individual carrier status. (A) Chromatogram of the MDH2 variant identified in 1 family from United States. (B) Pedigree of the family and MDH2 p.Arg52Cys individual carrier status. (C) Chromatogram of the MDH2 variant identified in 1 Italian family. (D) Pedigree of the Italian family and MDH2 p.Val160Met individual carrier status. Round and square symbols denote females and males, respectively. Filled and open symbols denote diabetic and nondiabetic subjects, respectively; half-filled symbols denote individuals with prediabetes (according to ADA 2014). Arrow points to proband. NM denotes presence of heterozygous MDH2 variant; NN denotes absence of such variant. The age at examination is reported for each individual under the corresponding symbol; the age at diagnosis is reported for diabetic or prediabetic individuals under the age at examination.
The structure and clinical features of the family are shown in Fig. 1B and Table 1. The age at onset of diabetes or prediabetes ranged from 2 to 58 years, with a median of 40 years. The 52Cys allele segregated with hyperglycemia, with 7 of the 8 affected family members available for genetic testing carrying the mutation and a deceased affected family member imputed to do so (Fig. 1B).
Table 1.
Clinical and genetic characteristics of examined members from the US and Italian families
| Family | Family member | Mutation carrier | Gender | Age (years) | BMI (kg/m2) | Glycemic status | Age at diagnosis (years) | Current treatment | FPG (mg/dL) | PG 2 hours after OGTT (mg/dL) | HbA1c (%) | Fasting insulin (mU/L) | HOMA-IR | HOMA-β |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| US | II-1 | Yes | M | 75 | 24.6 | DM | 40 | INS | 128 | NA | NA | NA | NA | NA |
| II-2 | No | F | 75 | 26.6 | NG | — | — | 98 | 114 | 5.6 | 12.0 | 2.9 | 123.4 | |
| III-1 | Yes | M | NA | NA | DM | 40 | INS | NA | NA | NA | NA | NA | NA | |
| III-2 | Yes | F | 49 | 21.7 | PD | 49 | — | 122 | 112 | 5.7 | 10.0 | 3.0 | 61.0 | |
| III-3 | No | F | 43 | 23.7 | NG | — | — | 96 | 114 | 5.4 | 6.0 | 1.4 | 65.4 | |
| III-4 | Yes | M | 42 | NA | DM | 35 | INS | 80 | NA | NA | NA | NA | NA | |
| III-5 | No | F | 43 | 23.0 | NG | — | — | 89 | 90 | 5.5 | 8.0 | 1,8 | 110.8 | |
| III-6 | No | F | 47 | 28.3 | NG | — | — | 92 | 139 | 5.3 | 9.0 | 2.0 | 111.7 | |
| III-7 | Yes | M | 56 | 27.4 | DM | 46 | INS | 103 | NA | NA | NA | NA | NA | |
| III-8 | Yes | F | 58 | 26.3 | PD | 58 | — | 113 | 112 | 5.9 | 9.0 | 2.5 | 64.8 | |
| IV-1 | Yes | M | 16 | 22.4 | NG | — | — | 92 | 76 | 5.4 | 6.0 | 1.4 | 74.5 | |
| IV-2 | Yes | F | 18 | 21.7 | DM | 2 | INS | 419 | NA | NA | NA | NA | NA | |
| IV-3 | No | F | 30 | 35.3 | PD | 30 | — | 109 | 158 | 5.7 | 13.0 | 3.5 | 101.7 | |
| Italian | IV-1 | No | F | 57 | 24.0 | PD | NA | — | 102 | 117 | 5.8 | 6.3 | 1.6 | 58.2 |
| IV-2 | Yes | M | 56 | 22.3 | DM | 45 | OADs | 102 | NA | 5.8 | 4.1 | NA | NA | |
| IV-3 | Yes | M | 62 | 22.0 | DM | 42 | INS | 155 | NA | 7.3 | NA | NA | NA | |
| IV-4 | No | F | 61 | 29.0 | PD | NA | — | 90 | 120 | 5.6 | 7.3 | 1.6 | 97.3 | |
| IV-5 | No | M | 63 | 26.0 | NG | — | — | 86 | 73 | 5.2 | 17.2 | 3.6 | 269.2 | |
| IV-6 | Yes | F | 53 | 21.0 | DM | 34 | INS | 104 | NA | 6.7 | NA | NA | NA | |
| IV-8 | No | F | 61 | 21.7 | NG | — | — | 74 | 122 | 5.4 | 5.4 | 1.0 | 176.7 | |
| IV-9 | Yes | F | 64 | 28.4 | NG | — | — | 75 | 135 | 5.2 | 8.3 | 1.5 | 249.0 | |
| V-1 | Yes | F | 30 | 28.0 | NG | — | — | 71 | 102 | 4.5 | 6.9 | 1.2 | 310.5 | |
| V-2 | Yes | M | 28 | 20.0 | NG | — | — | 93 | 122 | 4.6 | 7.4 | 1.7 | 88.8 | |
| V-3 | Yes | M | 34 | 23.7 | PD | 34 | — | 78 | 100 | 5.7 | 5.6 | 1.0 | 134.4 | |
| V-4 | No | M | 31 | 29.0 | NG | — | — | 87 | 88 | 4.7 | 4.6 | 1.0 | 69.0 | |
| V-5 | Yes | M | 23 | 21.0 | NG | — | — | 77 | 102 | 5.2 | 7.0 | 1.3 | 180.0 | |
| V-6 | Yes | F | 26 | NA | NG | — | — | 80 | 72 | 5.4 | 4.9 | 1.0 | 103.8 | |
| V-7 | Yes | F | 24 | 21.0 | NG | — | — | 73 | 80 | 5.1 | 4.4 | 0.8 | 158.4 | |
| V-8 | No | F | 14 | 18.7 | NG | — | — | 79 | 93 | 5.2 | 13.4 | 2.6 | 301.5 | |
| V-9 | No | M | 33 | 24.2 | NG | — | — | 80 | 57 | 5.2 | NA | NA | NA | |
| V-10 | No | F | 27 | 19.0 | NG | — | — | 70 | 80 | 5.1 | 5.0 | 0.9 | 257.1 |
HOMA-IR was computed as follows: fasting insulin (mU/L) × fasting glucose (mg/dL)/405. HOMA-β was calculated using the following formula: 360 × fasting insulin (mU/L)/fasting glucose (mg/dL) −63.
Abbreviations: BMI, body mass index; DM, diabetes mellitus; F, female; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; INS, insulin; M, male; NA, not available; NG, normal glucose; OADs, oral antidiabetes drugs; PD, prediabetes (as indicated by HbA1c >5.7%, according to ADA 2014 criteria (11)); HOMA, homeostasis model assessment.
The only affected individual (IV-3) who did not carry the mutation had prediabetes and a much higher BMI than affected carriers (Table 1), consistent with a phenocopy of typical T2D secondary to obesity. The Arg52Cys substitution was found in only 1 (IV-1) of the 5 normoglycemic members available for genetic testing, a 16-year-old boy who was probably still at risk of developing diabetes given the median age of diagnosis of the family (Fig. 1B).
MDH2 Variants in Additional Families
Looking for variants in MDH2 in the remaining families from the United States and in 8 additional families from Italy, for which WES data were also available, we found a second missense variant (c.478G>A; p.Val160Met) in 1 of the Italian families (Fig. 1C). Although this variant was observed in the general gnomAD database v.3.1.1 (MAF of 0.0003 in non-Finnish Europeans), it was absent from the “controls” dataset (N = 16 465 individuals). Based on this observation and the fact that no other variants identified by WES segregated with diabetes in this family (data not shown), the cosegregation of this variant with diabetes was also checked in the family.
The structure and clinical features of this family are shown in Fig. 1D and Table 1. The age at diagnosis of abnormal glucose homeostasis in the Italian family was similar to that in the US family (range and median of 34-45 and 39 years, respectively). Of the 15 members who were available for genetic testing, all 4 affected and 6 of the 11 nonaffected individuals were carriers of the Val160Met variant (Fig. 1D). Five of the 6 nonaffected carriers were younger than 34 years—the youngest age at diabetes diagnosis among affected members, raising the hypothesis of age-dependent penetrance of Val160Met (ie, penetrance becoming complete only at older age).
Contribution of MDH2 Rare Variants to Early-onset Diabetes
By focusing on MDH2 from WES data available for 2520 French individuals including 1144 patients with early-onset diabetes and 1376 normoglycemic controls, rare variants (ie, with MAF <0.001) in this gene showed a tendency to be enriched in patients with early-onset diabetes compared with nondiabetic subjects (5/1144 vs 1/1376, respectively; OR 6.03, P = .10).
MDH2 Expression and Correlation with Glucose-stimulated Insulin Release in Human Islets
According to the Genotype-Tissue Expression (GTEx, https://www.gtexportal.org/home/) database, MDH2 is expressed in several human tissues, including those playing central roles in glucose metabolism, such as skeletal muscle, liver, adipose tissue, endocrine pancreas, and brain. Therefore, we sought to explore the relationship between MDH2 mRNA levels and glucose-stimulated insulin release in human islets. To this end, MDH2 expression levels were measured in human islets from 7 nondiabetic and 5 diabetic brain-dead multi-organ donors. Compared with nondiabetic donors, MDH2 expression and glucose-stimulated insulin secretion, expressed as stimulation index, were both significantly lower in diabetic subjects than in the nondiabetic counterparts (P = .0006, Fig. 2A and P = .006, Fig. 2B, respectively). To further address the physiological role of MDH2 in insulin secretion in humans, the correlation between MDH2 expression levels and insulin stimulation index was assessed in an additional sample of 18 nondiabetic donors, in order to eliminate the possible confounding effect of hyperglycemia. In these samples, MDH2 expression was positively correlated with glucose-stimulated insulin release (r2 = 0.65, P = 5.3 × 10-5, Fig. 2C), thereby suggesting that MDH2 plays a critical role in insulin secretion.
Figure 2.
Human islets studies. (A) MDH2 mRNA expression level (calculated by using the ΔΔCT method) in islets from pancreata of nondiabetic and diabetic donors (*P = 0.0006, as assessed by Student’s t test). (B) Insulin stimulation index in nondiabetic and diabetic donors (*P = 0.006, as assessed by Student’s t test). (C) Relationship between stimulation index and MDH2 expression as evaluated by Pearson’s correlation (r2 = 0.65, P = 5.3 × 10-5 and P = 3.6 × 10–5, unadjusted and after adjusting for age and gender, respectively). Measurements showed in (A) and (B) were carried out on pancreatic islets prepared from pancreata of 7 nondiabetic and 5 diabetic brain-dead multiorgan donors while those showed in (C) were carried out on pancreatic islets prepared from pancreata of an additional 18 nondiabetic donors. Glucose-induced insulin secretion was measured and then expressed as stimulation index calculated by dividing insulin release after glucose stimulation at 16.7 mmol/L over basal insulin release (ie, at glucose 3.3 mmol/L).
In Silico Studies of MDH2 Variants and Dynamic Properties of the Mutant MDH2 Proteins
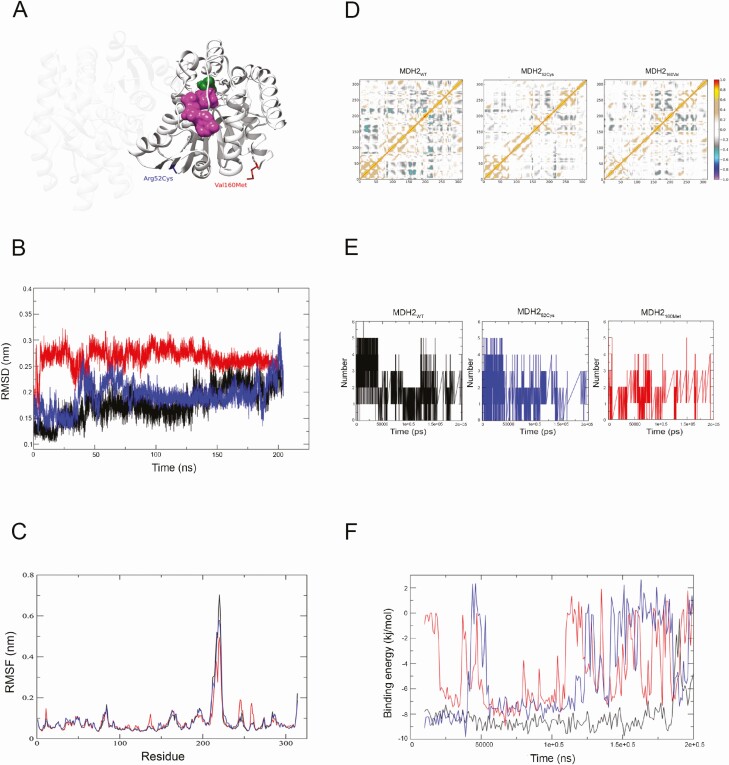
MDH2 encodes a mitochondrial enzyme that, by using the NAD+/NADH cofactor system, catalyzes the reversible oxidation of malate to oxaloacetate in the Krebs cycle (28) (also known as the tricarboxylic acid cycle, TCA). Together with glycolysis, the TCA plays a central role in the complete glucose breakdown and electron storage in NADH and is also involved in the malate-aspartate NADH shuttle (28). MDH2 acts as a homodimer, with each monomer containing a NAD-binding domain in the amino-terminal half, where Arg52Cys and Val160Met substitutions are located, and a malate-binding domain in the carboxy-terminal half (Fig. 3A). When evaluated by means of a previously reported scoring algorithm based on 13 in silico prediction tools (5), both substitutions were ranked as highly pathogenic, with estimated “pathogenicity scores” of 9/13 for Arg52Cys and 10/13 for Val160Met. To further investigate their possible functional impact, MD simulations were carried out. Conformational changes were assessed in terms of RMSD of all heavy atoms’ average distance. Compared with the wild-type protein (MDH2WT), both mutants (MDH252Cys and MDH2160Met) showed higher RMSD values through most of the simulation (Fig. 3B). Also, both mutated proteins showed altered flexibility in region spanning 213 to 235 amino acids, which includes part of the D-malate binding site (Fig. 3C). DCCMs also showed significant differences, with the anticorrelation movement of residues 17-55 vs residues 155-180 observed in MDH2WT being partially or entirely lost in MDH2160Met and MDH252Cys, respectively (Fig. 3D). Finally, the temporal pattern of hydrogen bonds established and destroyed during the simulation was quite different when comparing MDH252Cys or MDH2160Met to MDH2WT (Fig. 3E) and this was paralleled by higher binding energy levels in the 2 mutated proteins for almost the entire simulation (Fig. 3F). Taken together, all these in silico analyses suggested that both Arg52Cys and Val160Met substitution affects the MDH2 protein structure and function and motivated further experimental investigations.
Figure 3.
MDH2 structure and Molecular dynamics simulations. (A) Malate dehydrogenase catalyzes the reversible oxidation of malate (in green) to oxaloacetate, utilizing the NAD+/NADH cofactor system (NAD+/NADH in magenta) in the citric acid cycle. MDH2 protein exists as a dimer. Each subunit contains 2 structurally and functionally distinct domains. The first is the NAD-binding domain, located in the amino-terminal half of each molecule, and contains a parallel-sheet structure, otherwise known as a Rosman fold motif. The core dinucleotide binding structure is composed of 4 beta-sheets and 1 alpha-helix. The other domain is a carboxy-terminal domain containing the substrate binding site and amino acids necessary for catalysis. Arg52Cys and Val160Met amino acid changes are highlighted in blue and in red, respectively. (B) Instantaneous root mean square deviation (RMSD) of all heavy atoms. MDH2WT (in black), MDH252Cys (in blue), and MDH2160Met (in red). (C) Per-residue root mean square fluctuation as evaluated in MDH2WT (in black), MDH252Cys (in blue), and MDH2160Met (in red). (D) Dynamic cross correlation maps (DCCMs) as evaluated in MDH2WT, MDH252Cys, and MDH2160Met. (D) Peaks, corresponding to the Cij elements of the map, are indicative of strong to moderate positive correlation (red to brown), or of strong to moderate anti-correlation (violet to light gray) between the residues i and j of MDH2. (E) Hydrogen bonds as evaluated in MDH2WT (in black), MDH252Cys (in blue), and MDH2160Met (in red). (F) Binding energy between MDH2 and D-malate as a function of time. MDH2WT (in black), MDH252Cys (in blue), and MDH2160Met (in red).
MDH2 Enzymatic Activity and NAD+/NADH Ratio in HepG2 Transfected Cells
To experimentally verify the functional impact of the His52Cys and Val160Met variants, HepG2 human liver cells were transiently transfected with cDNAs coding for wild-type MDH2 (HepG2_MDH2WT), MDH252Cys (HepG2_MDH252Cys) or MDH2160Met (HepG2_MDH2160Met). MDH2 expression levels were not different across the 3 transfected cell lines (Fig. 4A). MDH2 enzymatic activity, as measured by quantifying the oxidation of malate to oxaloacetate, was markedly (approximately doubled) and significantly increased in cell lysates from HepG2_MDH2WT compared with those of untransfected cells (P < .0001, data not shown). Such activity was significantly higher in both HepG2_MDH252Cys and HepG2_MDH2160Met than in HepG2_MDH2WT cells throughout the entire 30-minute experiment (~26% of increase for both variants, P < .001, Fig. 4B). In addition, consistent with an increased MDH2 enzymatic activity of both mutants, HepG2_MDH252Cys and HepG2_MDH2160Met cells had lower NAD+/NADH ratios than HepG2_MDH2WT cells, although statistical significance was reached only for the 52Cys mutant (Fig. 4C). Overall, these studies in HepG2 cells suggest that both MDH2 Arg52Cys and Val160Met are gain-of-function alterations increasing MDH2 activity and causing ox–redox changes that have been shown to affect insulin signaling (29, 30) and secretion (31-33).
Figure 4.
MDH2 expression and enzymatic activity rate and NAD+/NADH ratio in HepG2 transfected cells. HepG2 cells were transiently transfected or not with V5-tagged MDH2WT or MDH2154 plasmids. After 48 hrs, cells were lysed to evaluate MDH2 expression, MDH2 enzymatic activity and NAD+/NADH ratio. (A) Equal amount of protein from cell lysates was separated by SDS-PAGE and analyzed by western blot with anti-β-actin (upper blot) and anti-MDH2 (lower blot) antibodies. Representative immunoblot is shown. A clear band of apparent molecular mass of 35 Kd, corresponding to the endogenous MDH2, was detectable in all cell lines. An additional band, of apparent molecular weight of 38 Kd, corresponding to the transfected MDH2, was visible in HepG2_MDH2WT, HepG2_ MDH252Cys, and HepG2_MDH2160Met. (B) MDH2 enzymatic activity was evaluated every 20 seconds for 30 minutes in 100 µg/mL of cell lysates. HepG2-subtracted enzymatic activity over time from HepG2_MDH2WT (black line), HepG2_ MDH252Cys (blue line) and HepG2_MDH2160Met (red line) cell lysates. Data are from 4 independent experiments performed in duplicate. *P < .001 as assessed by longitudinal linear model. (C) NAD+/NADH ratio on HepG2_MDH2WT (black bar), HepG2_ MDH252Cys (blue bar), and HepG2_MDH2160Met (red bar) cell lysates. Data are from 4 independent experiments performed in duplicate and expressed as percentage of NAD+/NADH ratio on HepG2 untransfected cells (means ± SD). *P < .05 as assessed by paired Student’s t test.
Impact of Wild-type and Mutant MDH2 on Glucose-stimulated Insulin Secretion
To verify the functional impact of the His52Cys and Val160Met variants on insulin secretion, mouse insulinoma MIN6-K8 cell lines were transduced with lentiviral constructs to stably express human MDH2WT, MDH252Cys, or MDH2160Met. MDH2 protein expression levels were not different across the 3 different cell lines (Fig. 5A). Compared with nontransduced cells, glucose-stimulated insulin secretion was 3-fold higher in cells expressing MDH2WT (Fig. 5B), thus reinforcing the hypothesis suggested by correlative data in human islets that MDH2 plays a positive role on glucose-stimulated insulin secretion. In contrast, a less pronounced increase was observed in cells expressing MDH252Cys or MDH2160Met (Fig. 5B) to the point that the difference vs nontransduced cells was no longer significant for MDH252Cys. Similar or even larger effects were observed for glucose plus 50 nM GLP-1-stimulated insulin secretion (Fig. 5B). When these data were expressed as fold increase relative to nontransduced cells, insulin secretion was significantly reduced in cells expressing either MDH252Cys or MDH2160Met compared with MDH2WT cells (Fig. 5B, inset).
Figure 5.
Glucose-stimulated insulin secretion in MIN6-K8 stably transfected cell lines. MIN6-K8 mouse insulinoma cells were transduced with lentiviral particles to stably express human MDH2WT, MDH252Cys, or MDH2160Met. Data are from 4 independent experiments. Means differences were assessed by Student’s t test. (A) A representative immunoblot is shown. Equal amount of protein from cell lysates was separated by SDS-PAGE and analyzed by western blot with antitubulin (upper blot) and anti-MDH2 (lower blot) antibodies. A clear band of apparent molecular mass of 35 Kd, corresponding to the endogenous MDH2 (mMDH2), was detectable in all cell lines. An additional band, of apparent molecular weight of 38 Kd, corresponding to the transduced human MDH2 (hMDH2), was visible in MIN6-K8_MDH2WT, _MDH252Cys, and _MDH2160Met (B) Glucose-stimulated insulin secretion (GSIS) was assessed under 2.8 and 16.7 mM glucose and 16.7 mM glucose plus 50 nM GLP-1. Bars show means ± SE; NS, not statistically significant; *P < .05 compared with nontransduced cells (NTCs). Inset shows insulin content expressed as fold increase with respect to NTCs (†P < .01 and *P < .05 compared with MIN6-K8_MDH2WT). Bars show means ± SD.
Impact of the Human MDH2 Alterations on Insulin Secretion in Genetically modified Caenorhabditis elegans
The C. elegans’ ortholog of human MDH2 (mdh-2) encodes a mitochondrial malate dehydrogenase displaying 60% of sequence identity and 76% of conservation with the human protein (Fig. 6A). The C. elegans genome encodes several insulin-like peptides (34). One of them, DAF-28, as mammalian insulin, is secreted and regulates metabolic homeostasis (26). Accordingly, its secretion is sensitive to nutritional status, being up- and downregulated by feeding and starvation, respectively (26). Similar to what is observed in mammals, an intact mitochondrial function is required to couple nutrient signals to DAF-28/insulin secretion (35). Since glucose-responsive pathways are conserved in this animal model (36), we first explored the effect of glucose on DAF-28/insulin secretion in wild-type worms and then evaluated the possible impact of both the mdh-2 variants in isogenic animals. To this end, strains carrying the mdh-2 mutant alleles as generated by CRISPR-Cas9, namely mdh-2(cer10[H56C]) and mdh-2(cer18[V164M]) (hereinafter mdh-256Cys and mdh-2164Met, respectively), were crossed with wild-type animals expressing a fluorescent reporter for the insulin-like peptide DAF-28 (pdaf-28::GFP), and baseline and glucose-stimulated insulin secretion were then investigated. As expected, in wild-type worms under baseline conditions, DAF-28 was expressed in ASI, ASJ, and PQR neurons, and the hind gut, and was secreted into the pseudocoelomic fluid, where it was internalized by coelomocytes (26) (Fig. 6B). Interestingly, in these animals, DAF-28 secretion was significantly increased after glucose stimulation (Fig. 6C). By contrast, compared with controls, both mdh-256Cys and mdh-2164Met animals showed a striking increase in baseline DAF-28 secretion, along with a blunted ability to further increase this in response to glucose stimulation (Fig. 6C). Accordingly, delta DAF-28 fluorescence (glucose stimulated minus baseline) was significantly lower in mutants than in control animals (P < .001). These findings closely resemble the phenotype of patients with T2D, who at diagnosis show normal or increased fasting insulin levels along with a marked reduction in glucose-stimulated insulin secretion (37). In addition, mdh-2 mutant animals also exhibited a higher prevalence of glucose-induced egg retention with internal hatching events (Bag-of-worms phenotype) (38, 39) compared with controls (Fig. 6D), thus suggesting glucose accumulation in the former.
Figure 6.
Caenorhabditis elegans studies. (A) Sequence alignment of human MDH2 and the C. elegans MDH-2 ortholog protein indicating conservation between species. The affected residues are shown in bold and red. Identity and conservation (+) of individual residues is also reported (middle row). (B,C) Basal and glucose-stimulated DAF-28/insulin secretion. pdaf-28∷GFP expression in control and mutant adult hermaphrodites grown in NGM or in the presence of 30 mM D-glucose. DAF-28 is expressed in 2 bilaterally symmetric pairs of sensory neurons of the head (ASI and ASJ) containing projections extended to the tip of the nose and others joining the nerve ring, the tail neuron PQR, and the hind gut cells. The GFP amount in coelomocytes is used to measure secreted DAF-28/insulin. The mean fluorescence intensity was calculated using the NIH Image J software (right panel). *P < .005 and **P < .001 by Student’s t-test compared with basal DAF-28 expression observed in pdaf-28∷GFP animals. pdaf-28∷GFP (basal, n = 83; +glucose, n = 56); mdh-256Cys;pdaf-28∷GFP (basal, n = 30; +glucose, n = 30); mdh-2164Met;pdaf-28∷GFP (basal, n = 40; +glucose, n = 56). (D) Egg retention and internal hatching (Bag-of-worms phenotype) in animals grown in the presence of 112 mM D-glucose (*P < .002, Fisher exact test). Control (n = 66), mdh-256Cys (n = 55); mdh-2164Met (n = 46).
Discussion
The present study is part of our ongoing effort to understand the as yet unknown genetic causes of diabetes in multigenerational families with autosomal dominant forms of adult diabetes not due to mutations in known monogenic diabetes genes (4, 5, 40). Here we report 2 heterozygous missense variants—a very rare missense mutation (p.Arg52Cys) and an infrequent missense substitution (p.Val160Met)—in the gene coding for MDH2. Both variants behave as gain-of-function alterations segregating with hyperglycemia in 2 different pedigrees and affecting insulin secretion, thereby pointing to MDH2 as a possible new disease gene. Interestingly, the median age at diagnosis in affected members from both families was approximately 40 years, thus supporting the concept that such forms of familial diabetes might be intermediate forms of hyperglycemia between MODY and T2D (4, 5). In agreement with this observation, one-third of affected individuals in both families were overweight—an uncommon condition in MODY patients but an almost ineludible feature of patients with T2D.
Several lines of evidence from in silico analyses, in vitro studies with human and mouse cells, and in vivo experiments with genetically modified worms, provide robust support for a pathogenic role of the MDH2 Arg52Cys variant in dysregulating glucose homeostasis and insulin secretion. Firstly, in depth in silico analyses and simulations clearly suggested that the Arg52Cys substitution affects MDH2 protein structure and function. Secondly, the amino acid change behaved as a gain-of-function alteration in transfected human liver cells, significantly increasing MDH2 enzymatic activity. In agreement with these findings, a decreased NAD+/NADH ratio—a redox change known to be deleterious for both insulin signaling and secretion—was observed in cell lines carrying this mutation. Thirdly, data obtained in mouse insulinoma MIN6-K8 cells clearly showed that stable expression of the MDH2 mutations cause a blunted glucose- and glucose plus GLP-1-stimulated insulin secretion. Finally, C. elegans carrying the Arg52Cys mutation at the orthologous positions of the nematode gene showed a blunted glucose-stimulated insulin secretion similar to that observed in MIN6-K8 cells stably expressing the human MDH2 mutants. All these observations strongly support a role of the Arg52Cys variant as a disease-causing mutation in the family in which it was identified.
Similar evidence was obtained for the Val160Met substitution found in the Italian family. However, some caution is needed in interpreting the role of this variant given the weaker in vitro and in vivo functional impact, the incomplete and age-dependent penetrance, and the less rare allelic frequency, pointing to Val160Met as a “hypomorphic mutation,” for which combination with other genetic or environmental factors is necessary to cause overt diabetes in this family. Of note, hypomorphic variants have already been reported in some MODY genes as being responsible for reduced and age-dependent penetrance in a family context (41-43), in conjunction with a unexpectedly high frequency in public variant databases (41, 43).
Finally, MDH2 rare variants (ie, with MAF < 0.001) showed a tendency to be enriched in patients with early-onset diabetes compared with nondiabetic subjects, further supporting a role of MDH2 gene in affecting glucose homeostasis. Of note, rare variants in genes responsible for different monogenic forms of diabetes (namely ND and MODY) have also been associated (singly or collectively) with an increased risk of common T2D (44-48) or early onset diabetes (49, 50).
Altogether, these preliminary findings, although to be confirmed in larger family collections, are consistent with the hypothesis of an MDH2 role in glucose homeostasis. However, despite all these pieces of evidence, the intimate mechanism linking the MDH2 gain-of-function mutations to hyperglycemia remains unclear. MDH2 expression was positively correlated with insulin secretion in islets from nondiabetic individuals, and was decreased in islets from patients with T2D. Also, stable expression of human wild-type MDH2 in MIN6 cells increased insulin secretion. In light of these observations, the fact that gain-of-function MDH2 mutations have diabetogenic effects may seem counterintuitive. One possibility is that the constitutive hyperactivity of the malate–aspartate shuttle, and the resulting chronic stimulation of insulin secretion triggered by the MDH2 gain-of-function mutation causes β cell desensitization and defective insulin secretion as it happens after prolonged β cell stimulation by pharmacological (eg, sulfonylureas) or physiological (eg, nutrients) secretagogues (51). Another possibility is that the reduction in the NAD+/NADH ratio resulting from the gain-of-function MDH2 mutation and the resulting MDH2 overactivity, when it exceeds a certain level, offsets the beneficial effects on insulin secretion caused by the activation of the malate–aspartate shuttle. The NAD+/NADH ratio is a key regulator of the cell’s redox state (52) and energy metabolism, which is especially relevant to pancreatic β cells where glycolysis and oxidative phosphorylation serve as the coupling mechanisms between blood glucose and insulin secretion (53-55). Thus, a NAD+/NADH reduction, as that attributable to the MDH2 gain-of-function alterations, has the potential to affect insulin secretion (56) and has been repeatedly implicated in the etiology of diabetes (57). It is also possible that the combination of these 2 effects of MDH2 hyperactivity, namely chronic stimulation of insulin secretion in the presence of a reduced mitochondrial NAD+/NADH, is especially deleterious for β-cell physiology.
Of note, biallelic MDH2 loss-of-function mutations have been recently reported to cause an autosomal recessive form of early-onset severe encephalopathy. No alterations of glucose homeostasis have been reported in affected cases (58). Whether this is due to the existence of compensatory mechanisms for MDH2 deficiency in β cells, or to the fact that the metabolic abnormalities caused by loss-of-function mutations are subtle and difficult to detect in the early years of life, remains to be established.
In summary, we have identified heterozygous missense, gain-of-function variants in the MDH2 gene showing evidence of cosegregation with hyperglycemia in families with autosomal dominant diabetes. The data concerning the effect of these alterations in cell lines and C. elegans point to MDH2 as a potential key node in the regulation of glucose homeostasis. Further genetic and mechanistic studies are warranted to confirm MDH2 as a diabetogene, to understand the precise mechanisms through which MDH2 mutations cause diabetes, and to determine whether MDH2 could be a viable target for the development of new treatments of hyperglycemia.
Acknowledgments
We wish to thank the subjects and families involved in the study, the WormBase and Dr. Maria Giovanna Scarale (Fondazione IRCCS Casa Sollievo della Sofferenza) for supporting statistical analyses in C. elegans and in HepG2 cell experiments. We also thank the Caenorhabditis Genetics Center. S. Prudente and A.D. are the guarantors of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Support: This work was supported by the Italian Diabetes Society (SID) (grant FO.RI.SID 2011 to S. Prudente and 2019 to V.T.), by NIH (grant R01DK55523 to A.D. and P30 DK036836 to the Joslin Diabetes Research Center [Advanced Genomics and Genetics Core]), by the Italian Ministry of Health (Ricerca Corrente 2018-2021 to S. Prudente, R.D.P., V.T., T.B., and T.M.), by the Italian Ministry of University and Research (PRIN 2016 to V.T.), by voluntary contribution to Fondazione IRCCS Casa Sollievo della Sofferenza (“5x1000”), by Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand (grant R016034011 to P.J.T.) and by the Thailand Research Fund and Office of the Higher Education Commission, Thailand (grant MRG6280107 to P.J.T.). T.B. and T.M. are supported by NVIDIA corporation. The Caenorhabditis Genetics Center is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Author Contributions: S. Prudente and A.D. conceived, designed and supervised the study and wrote the manuscript. P.J.T. performed WES experiments and genetic studies and helped writing the manuscript. S. Pezzilli, L.M., F.A., C.M., T.H., and M.C. performed genetic analyses. A.M., E.F., R.D.P. and Z.A, performed cell culture experiments. L. Marselli and P.M. performed studies in human islets. T.B. and T.M. performed bioinformatic analyses and molecular dynamics simulations.
M.P.D.L.R. and J.C. performed CRISPR/Cas9 genome editing on C. elegans strains. L.P. and S.M. designed and performed C. elegans in vivo studies. O.L. performed clinical characterization of Italian families. P.B. performed clinical characterizations of US families. A.B. and P.F. performed data curation and statistical analysis in WES from French individuals, V.T. supervised both cell culture experiments and clinical characterization of Italian families and wrote the manuscript. All authors approved the final manuscript and contributed critical revisions to its intellectual content.
Glossary
Abbreviations
- BMI
body mass index
- DCCM
dynamic cross-correlation map
- KRB
Krebs–Ringer bicarbonate
- MAF
minor allele frequency
- MDH2
malate dehydrogenase 2
- MODY
maturity onset diabetes of the young
- ND
neonatal diabetes
- NGM
normal growth medium
- RMSD
root mean square deviation
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- WES
whole-exome sequencing
Additional Information
Conflict of Interest: No potential conflicts of interest relevant to this article were reported.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13- S27. [DOI] [PubMed] [Google Scholar]
- 3. Vaxillaire M, Froguel P. Monogenic diabetes: implementation of translational genomic research towards precision medicine. J Diabetes. 2016;8(6):782-795. [DOI] [PubMed] [Google Scholar]
- 4. Ludovico O, Carella M, Bisceglia L, et al. Identification and clinical characterization of adult patients with multigenerational diabetes mellitus. PLoS One. 2015;10(8):e0135855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pezzilli S, Ludovico O, Biagini T, et al. Insights from molecular characterization of adult patients of families with multigenerational diabetes. Diabetes. 2018;67(1):137-145. [DOI] [PubMed] [Google Scholar]
- 6. Prudente S, Jungtrakoon P, Marucci A, et al. Loss-of-function mutations in APPL1 in familial diabetes mellitus. Am J Hum Genet. 2015;97(1):177-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jungtrakoon P, Shirakawa J, Buranasupkajorn P, et al. Loss-of-function mutation in thiamine transporter 1 in a family with autosomal dominant diabetes. Diabetes. 2019;68(5):1084-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doria A, Yang Y, Malecki M, et al. Phenotypic characteristics of early-onset autosomal-dominant type 2 diabetes unlinked to known maturity-onset diabetes of the young (MODY) genes. Diabetes Care. 1999;22(2):253-261. [DOI] [PubMed] [Google Scholar]
- 9. American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81-S90. [DOI] [PubMed] [Google Scholar]
- 10. Carr IM, Camm N, Taylor GR, et al. GeneScreen: a program for high-throughput mutation detection in DNA sequence electropherograms. J Med Genet. 2011;48(2):123-130. [DOI] [PubMed] [Google Scholar]
- 11. Marchetti P, Suleiman M, Marselli L. Organ donor pancreases for the study of human islet cell histology and pathophysiology: a precious and valuable resource. Diabetologia. 2018;61(4):770-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marchetti P, Bugliani M, Lupi R, et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007;50(12):2486-2494. [DOI] [PubMed] [Google Scholar]
- 13. Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Biagini T, Chillemi G, Mazzoccoli G, et al. Molecular dynamics recipes for genome research. Brief Bioinform. 2018;19(5):853-862. [DOI] [PubMed] [Google Scholar]
- 15. Pettersen EF, Goddard TD, Huang CC, et al. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605-1612. [DOI] [PubMed] [Google Scholar]
- 16. Darden T, York D, Pedersen L. Particle mesh Ewald: AN N⋅log(N) method for Ewald sums in large systems. J Chem Phys. 1993;98:10089. [Google Scholar]
- 17. Feller SE, Zhang Y, Pastor W. Constant pressure molecular dynamics simulation: the Langevin piston method. J Chem Phys. 1995;103:4613. [Google Scholar]
- 18. Pronk S, Páll S, Schulz R, et al. GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics. 2013;29(7):845-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marucci A, Cozzolino F, Dimatteo C, et al. Role of GALNT2 in the modulation of ENPP1 expression, and insulin signaling and action: GALNT2: a novel modulator of insulin signaling. Biochim Biophys Acta. 2013;1833(6):1388-1395. [DOI] [PubMed] [Google Scholar]
- 20. Iwasaki M, Minami K, Shibasaki T, Miki T, Miyazaki J, Seino S. Establishment of new clonal pancreatic β-cell lines (MIN6-K) useful for study of incretin/cyclic adenosine monophosphate signaling. J Diabetes Investig. 2010;1(4):137-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murphy CT, Hu PJ. Insulin/insulin-like growth factor signaling in C. elegans. WormBook. 2013:1-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Porte D Jr, Baskin DG, Schwartz MW. Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes. 2005;54(5):1264-1276. [DOI] [PubMed] [Google Scholar]
- 23. Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov. 2006;5(5):387-398. [DOI] [PubMed] [Google Scholar]
- 24. Sulston JE, Hodgkin J. Methods. In: Wood WB, ed. The Nematode Caenorhabditis Elegans. Cold Spring Harbor Laboratory Press; 1988:587-606. [Google Scholar]
- 25. Paix A, Folkmann A, Rasoloson D, Seydoux G. High efficiency, homology-directed genome editing in Caenorhabditis elegans using CRISPR-Cas9 ribonucleoprotein complexes. Genetics. 2015;201(1):47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li W, Kennedy SG, Ruvkun G. daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes Dev. 2003;17(7):844-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Richards S, Aziz N, Bale S, et al. ; ACMG Laboratory Quality Assurance Committee . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Minárik P, Tomásková N, Kollárová M, Antalík M. Malate dehydrogenases–structure and function. Gen Physiol Biophys. 2002;21(3):257-265. [PubMed] [Google Scholar]
- 29. Cheng Z, Tseng Y, White MF. Insulin signaling meets mitochondria in metabolism. Trends Endocrinol Metab. 2010;21(10):589-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect. 2015;4(1):R1-R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dukes ID, McIntyre MS, Mertz RJ, et al. Dependence on NADH produced during glycolysis for beta-cell glucose signaling. J Biol Chem. 1994;269(15):10979-10982. [PubMed] [Google Scholar]
- 32. Eto K, Tsubamoto Y, Terauchi Y, et al. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science. 1999;283(5404):981-985. [DOI] [PubMed] [Google Scholar]
- 33. Fex M, Nicholas LM, Vishnu N, et al. The pathogenetic role of β-cell mitochondria in type 2 diabetes. J Endocrinol. 2018;236(3):R145-R159. [DOI] [PubMed] [Google Scholar]
- 34. Husson SJ, Mertens I, Janssen T, Lindemans M, Schoofs L. Neuropeptidergic signaling in the nematode Caenorhabditis elegans. Prog Neurobiol. 2007;82(1):33-55. [DOI] [PubMed] [Google Scholar]
- 35. Billing O, Kao G, Naredi P. Mitochondrial function is required for secretion of DAF-28/insulin in C. elegans. PLoS One. 2011;6(1):e14507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mondoux MA, Love DC, Ghosh SK, et al. O-linked-N-acetylglucosamine cycling and insulin signaling are required for the glucose stress response in Caenorhabditis elegans. Genetics. 2011;188(2):369-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595-1607. [DOI] [PubMed] [Google Scholar]
- 38. Teshiba E, Miyahara K, Takeya H. Glucose-induced abnormal egg-laying rate in Caenorhabditis elegans. Biosci Biotechnol Biochem. 2016;80(7):1436-1439. [DOI] [PubMed] [Google Scholar]
- 39. Wu J, Jin Z, Zheng H, Yan LJ. Sources and implications of NADH/NAD(+) redox imbalance in diabetes and its complications. Diabetes Metab Syndr Obes. 2016;9:145-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prudente S, Ludovico O, Trischitta V. Familial diabetes of adulthood: A bin of ignorance that needs to be addressed. Nutr Metab Cardiovasc Dis. 2017;27(12):1053-1059. [DOI] [PubMed] [Google Scholar]
- 41. Laver TW, Colclough K, Shepherd M, et al. The common p.R114W HNF4A mutation causes a distinct clinical subtype of monogenic diabetes. Diabetes. 2016;65(10):3212-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patel KA, Kettunen J, Laakso M, et al. Heterozygous RFX6 protein truncating variants are associated with MODY with reduced penetrance. Nat Commun. 2017;8(1):888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wright CF, West B, Tuke M, et al. Assessing the pathogenicity, penetrance, and expressivity of putative disease-causing variants in a population setting. Am J Hum Genet. 2019;104(2):275-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jafar-Mohammadi B, Groves CJ, Gjesing AP, et al. ; DIAGRAM Consortium . A role for coding functional variants in HNF4A in type 2 diabetes susceptibility. Diabetologia. 2011;54(1):111-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Estrada K, Aukrust I, Bjørkhaug L, et al. ; SIGMA Type 2 Diabetes Consortium . Association of a low-frequency variant in HNF1A with type 2 diabetes in a Latino population. JAMA. 2014;311(22):2305-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Najmi LA, Aukrust I, Flannick J, et al. Functional investigations of HNF1A identify rare variants as risk factors for type 2 diabetes in the general population. Diabetes. 2017;66(2):335-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Flannick J, Beer NL, Bick AG, et al. Assessing the phenotypic effects in the general population of rare variants in genes for a dominant Mendelian form of diabetes. Nat Genet. 2013;45(11):1380-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fuchsberger C, Flannick J, Teslovich TM, et al. The genetic architecture of type 2 diabetes. Nature. 2016;536(7614):41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hegele RA, Cao H, Harris SB, Hanley AJ, Zinman B. The hepatic nuclear factor-1alpha G319S variant is associated with early-onset type 2 diabetes in Canadian Oji-Cree. J Clin Endocrinol Metab. 1999;84(3):1077-1082. [DOI] [PubMed] [Google Scholar]
- 50. Pezzilli S, Tohidirad M, Biagini T, et al. Contribution of rare and common genetic variants to early-onset type 2 diabetes. Diabetologia. 2020;63(Suppl 1):S170. [Google Scholar]
- 51. Ball AJ, Flatt PR, McClenaghan NH. Desensitization of sulphonylurea- and nutrient-induced insulin secretion following prolonged treatment with glibenclamide. Eur J Pharmacol. 2000;408(3):327-333. [DOI] [PubMed] [Google Scholar]
- 52. Xiao W, Wang RS, Handy DE, Loscalzo J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid Redox Signal. 2018;28(3):251-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18(2):162-185. [DOI] [PubMed] [Google Scholar]
- 54. Maechler P, Carobbio S, Rubi B. In beta-cells, mitochondria integrate and generate metabolic signals controlling insulin secretion. Int J Biochem Cell Biol. 2006;38(5-6):696-709. [DOI] [PubMed] [Google Scholar]
- 55. MacDonald PE, Joseph JW, Rorsman P. Glucose-sensing mechanisms in pancreatic beta-cells. Phil Trans R Soc Lond B Biol Sci. 2005;360(1464):2211-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jitrapakdee S, Wutthisathapornchai A, Wallace JC, MacDonald MJ. Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia. 2010;53(6):1019-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bender K, Maechler P, McClenaghan NH, Flatt PR, Newsholme P. Overexpression of the malate-aspartate NADH shuttle member Aralar1 in the clonal beta-cell line BRIN-BD11 enhances amino-acid-stimulated insulin secretion and cell metabolism. Clin Sci (Lond). 2009;117(9):321-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ait-El-Mkadem S, Dayem-Quere M, Gusic M, et al. Mutations in MDH2, encoding a krebs cycle enzyme, cause early-onset severe encephalopathy. Am J Hum Genet. 2017;100(1):151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.