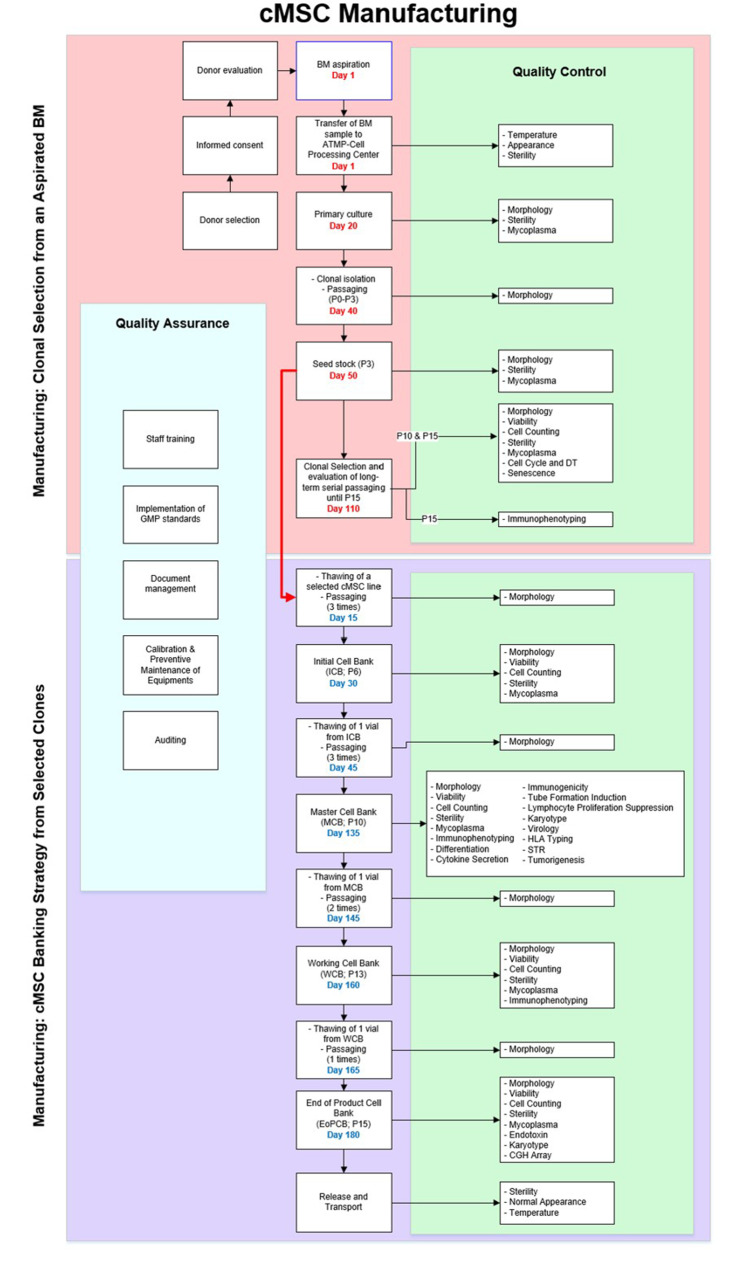
Fig. 3.
Process flow diagram for GMP-grade manufacturing of cMSCs and the necessary QC assessments. Schema indicated the master production schedule for BM sampling, the manufacturing steps, and biobanking concerning the passage numbers and QC tests. The approximate timescale for the main production stages from BM sampling to seed stock establishment as well as clone selection process is written in red. The timescale for establishment of the four tiered cell bank (written in blue) is started from the seed stock of the selected clones. The minimum time needed for production and QC assessments were considered for estimating the timescale. GMP: Good manufacturing practice; BM: Bone marrow; cMSCs: Clonal mesenchymal stromal cells; QC: Quality control; ICB: Initial cell bank, MCB: Master cell bank, WCB: Working cell bank, EoPCB: End of product cell bank, IPQC: In-process quality control, GLP: Good laboratory practice