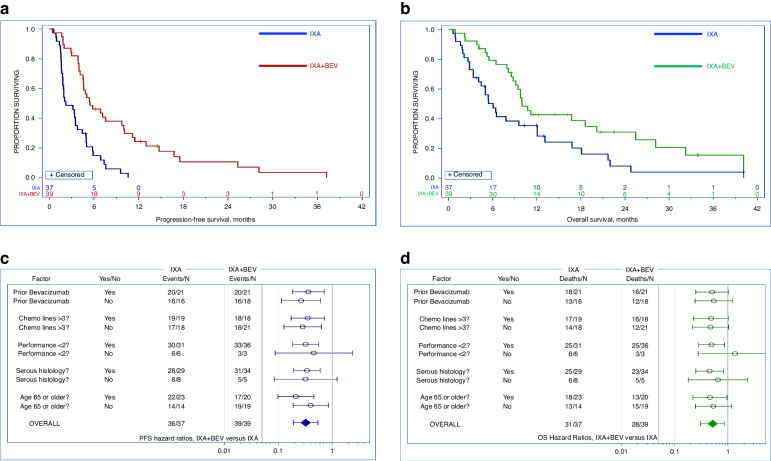
Fig. 2. Progression-free survival, overall survival, and subgroup analyses.
a Progression-free survival: use of bevacizumab (BEV) with ixabepilone (IXA) significantly extended progression-free survival (5.5 vs 2.2 months, HR 0.33, 95% CI 0.19–0.55, p < 0.001) compared to IXA alone. b Overall Survival: overall survival was significantly longer in patients who received BEV in conjunction wiht IXA (10.0 vs 6.0 months, HR 0.52, 95% CI 0.31–0.87, p < 0.006). c Hazard ratios for progression-free survival versus treatment arm by subgroup: progression-free survival hazard radios were similar between arms among patients with prior BEV exposure (HR 0.36, 95% CI: 0.19–0.72, p = 0.003) and those who were BEV-naive (HR 0.27, 95%CI: 0.12–0.62, p = 0.002). Stratification by pre-treatment status, age, histology, and performance status are also shown. Error bars denote 95% confidence intervals (CI). d Hazard ratios for overall survival versus treatment arm by subgroup: similar hazard ratios for overall survival were observed between arms among patients with prior BEV exposure (HR 0.50, 95%: 0.25–1.02, p = 0.058) and those who were BEV-naive (HR 0.54, 95% CI: 0.24–1.22, p = 0.14). Stratification by pre-treatment status, age, histology, and performance status are also shown. Error bars denote 95% CI.