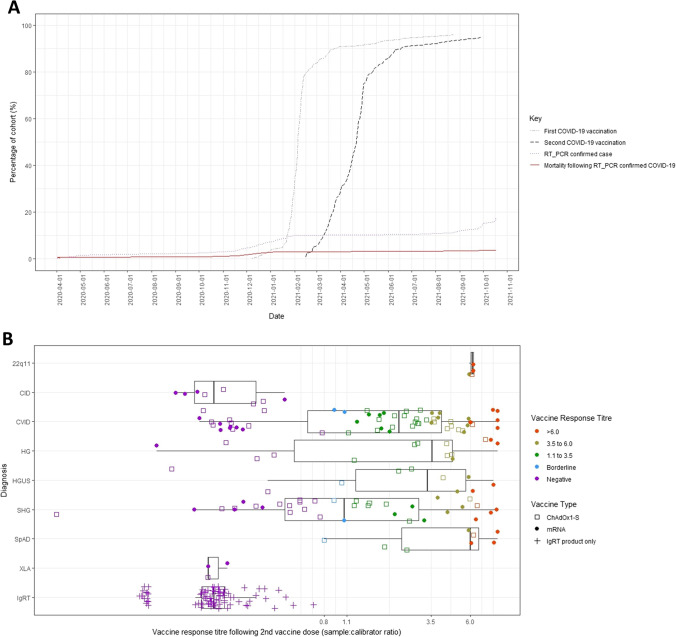
Fig. 1.
Uptake and serological response following 2 doses of COVID-19 vaccination in adults under care of the Immunodeficiency Centre for Wales. A Uptake of first (grey, dot-dashed) and second (black, dashed) COVID-19 vaccination; cumulative total of patient cohort with molecularly confirmed SARS-CoV-2 infection (purple, dotted) and subsequent mortality (red, solid). Shielding of clinically extremely vulnerable individuals in Wales was implemented between March and August 2020, directing such individuals to stay at home to protect themselves. B Anti-SARS-CoV-2 spike IgG serum responses elicited by 2 doses of COVID-19 vaccination in individuals under care of the Immunodeficiency Centre for Wales (ICW) assayed using the semi-quantitative EUROIMMUN IgG assay. Vaccine response indicated on the x-axis by sample:calibrator ratio. Titre grading shown reflects assay cut-off and reported criteria used for selection of convalescent plasma therapy at that time. Patients are sub-grouped by clinical diagnosis (22q11- DiGeorge 22q11 deletion syndrome; CID- Combined Immunodeficiency (without defined molecular diagnosis, including Good’s syndrome); CVID- common variable immunodeficiency syndrome; HG- hypogammaglobulinaemia (insufficient to meet criteria for CVID); HGUS- hypogammaglobulinaemia of uncertain significance (not requiring immunoglobulin replacement therapy); SHG- secondary hypogammaglobulinaemia; SpAD- specific antibody deficiency; XLA- X-linked agammaglobulinaemia; IgRT- immunoglobulin replacement therapy products, diluted to simulate in vivo infusion at replacement dosing). Vaccine type indicated by open squares (ChAdOx1-S, AstraZeneca) and filled circles (mRNA, Pfizer)