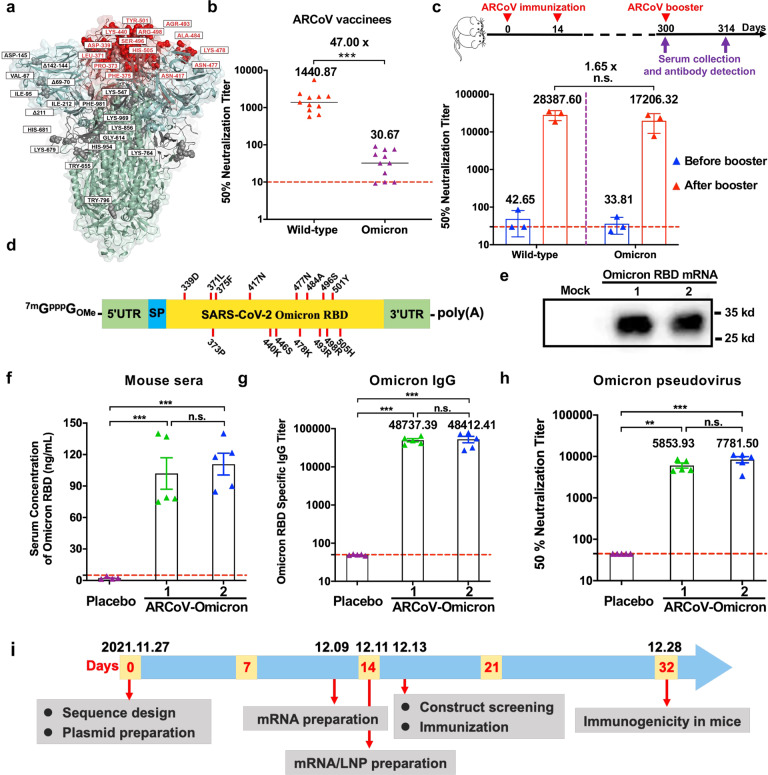
Fig. 1. Development and characterization of an updated RBD-based mRNA vaccine against Omicron.
a Structural model of amino acid mutations in the Spike protein of the Omicron variant of SARS-CoV-2. The model was built using SWISS-MODEL15 with a structure of SARS-CoV-2 spike protein (PDB: 6 ZGE16) as the template model and visualized with PyMOL(v2.5.0). The RBD, NTD, and S2 region of Spike protein were colored by red, blue and green, respectively. All mutations in the RBD were highlighted in darker color, and mutations or deletions in other region of Spike protein were colored in gray. b The 50% neutralization titer of sera from ARCoV vaccinees (n = 11) were analyzed using VSV-based pseudovirus for WT and Omicron, respectively. The red dashed lines indicate the detection limit of the assay. Data are shown as means ± SEM and analyzed using unpaired t-test (***P < 0.001). c Neutralization assay after a booster ARCoV vaccination in mice. Groups of 8–9-month female BALB/c mice were intramuscularly immunized and boosted with 10 μg ARCoV (n = 3), and sera were detected at the indicated time points. The red dashed lines indicate the detection limit of the assay. Data are shown as means ± SEM and analyzed using a one-way ANOVA with multiple comparisons tests (n.s., not significant). d Schematic representation of the mRNA construct encoding Omicron RBD. The mutation sites were indicated with red lines. e Omicron RBD protein expression from mRNA in Huh-7 cells. Cells were transfected with Omicron RBD-encoding mRNAs, and the supernatants was detected by Western blotting assay 24 h after transfection. f In vivo expression of ARCoV-Omicron in mice. Groups of 6–8-week female ICR mice (n = 5) were intravenously inoculated with ARCoV-Omicron, and PBS (n = 4) was used as Placebo. The serum concentration of Omicron RBD was measured by ELISA. Data are shown as means ± SEM and analyzed using one-way ANOVA with multiple comparisons tests (n.s., not significant; ***P < 0.001). g, h The immunogenicity of ARCoV-Omicron in mice. Groups of 6–8-week female ICR mice (n = 5) were immunized intramuscularly with two doses of 10 μg of ARCoV-Omicron at 7-day interval. Sera were collected at 14 days after initial immunization and subjected to IgG and neutralization antibody assays. The red dashed lines indicate the detection limit of the assay. Data are shown as means ± SEM and analyzed using a one-way ANOVA with multiple comparisons tests (**P < 0.01, ***P < 0.001). i Timeline for the rapid development of ARCoV-Omicron mRNA vaccine.