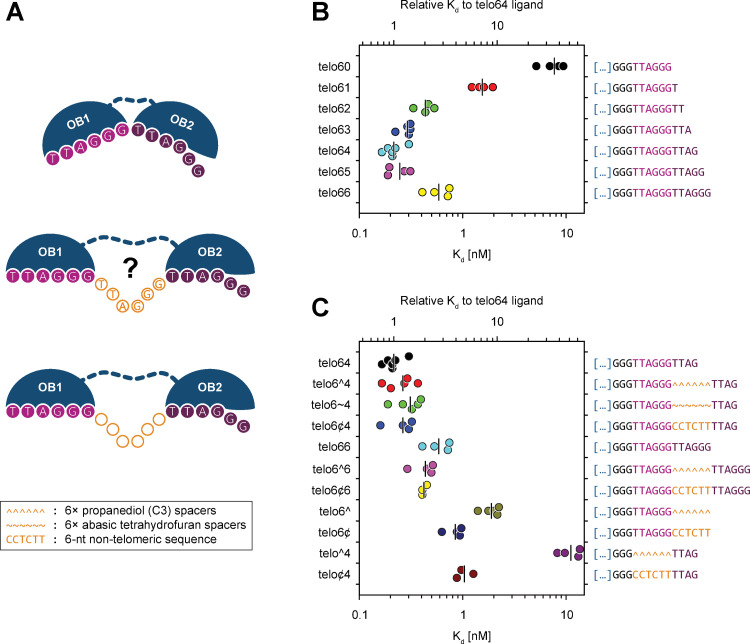
Fig 6. EMSA demonstrate that binding of telomeric ssDNA to the POT1 OB1/OB2 domain is not limited to the minimal binding sequence, but to sequences separated by spacers as well.
(A) Cartoon representation of the POT1 OB1/2 domains and its possible binding conformations that can allow binding to the minimal tight binding sequence TTAGGGTTAG (top), or the same sequence separated by either a single telomeric repeat (center) or a ~6-nt long non-telomeric spacer (bottom). (B) Horizontal scatter plot with median marking of the apparent binding affinity of various telomeric ligands for the POT1-TPP1-TIN2(1–354) subcomplex. Each individual Kd value was computed from the EMSA data shown in S6B–S6H Fig. The binding experiments confirm that telo64 is truly the minimal tight-binding sequence. Both shorter and longer oligos, with the notable exception of telo65 ligand, present reduced affinities for the subcomplex. (C) Horizontal scatter plot with median marking of the apparent binding affinity of various non-contiguous telomeric ligands for the POT1-TPP1-TIN2(1–354) subcomplex. Each individual Kd value was computed from the EMSA data shown in S6I–S6Q Fig. The binding experiments show that the minimal tight-binding sequence ligands that have been interrupted with various non-telomeric spacers retain binding affinity comparable to that of the contiguous telo64 ligand.