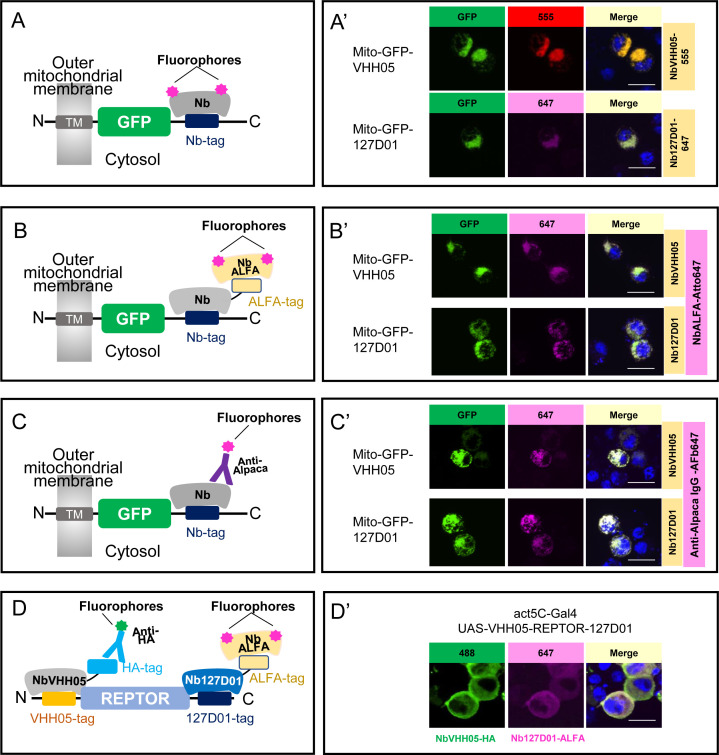
Figure 2. Using NbVHH05 and Nb127D01 for immunofluorescence.
(A) Fluorophore-conjugated NbVHH05 or Nb127D01 recognizes VHH05- or 127D01-tagged fluorescence proteins. (A’) VHH05- or 127D01-tagged mito-GFP can be detected by the corresponding NbVHH05-555 or Nb127D01-647 in transfected S2R+ cells. 4′,6-Diamidino-2-phenylindole (DAPI) staining shows the nuclei. (B) Schematic of nanobodies containing ALFA-tag as primary antibody and NbALFA as a secondary antibody. (B’) VHH05- or 127D01-tagged mito-GFP can be detected using the corresponding nanobodies in transfected S2R+ cells. (C) Schematic of fluorophore-conjugated anti-Alpaca IgG antibodies to detect VHH05- and 127D01-tagged proteins. NbVHH05 or Nb127D01 is used as primary antibodies and anti-Alpaca IgG as secondary antibody. (C’) VHH05- or 127D01-tagged mito-GFP can be detected using the corresponding nanobodies and anti-Alpaca IgG-647 in transfected S2R+ cells. (D) Schematic of using VHH05 and 127D01 for double tagging. N-, C-terminal of REPTOR contains VHH05 and 127D01. (D’) Co-staining NbVHH05 and Nb127D01 in S2R+ cells transfected with VHH05-REPTOR-127D01. Scale bars: 10 µm.