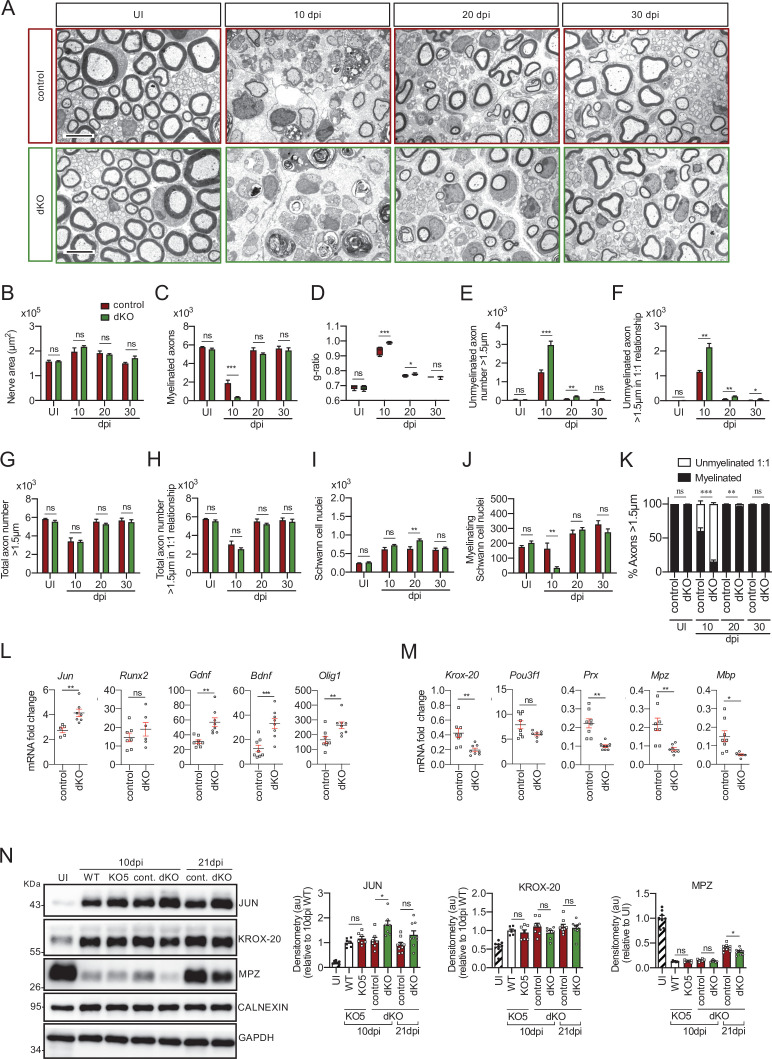
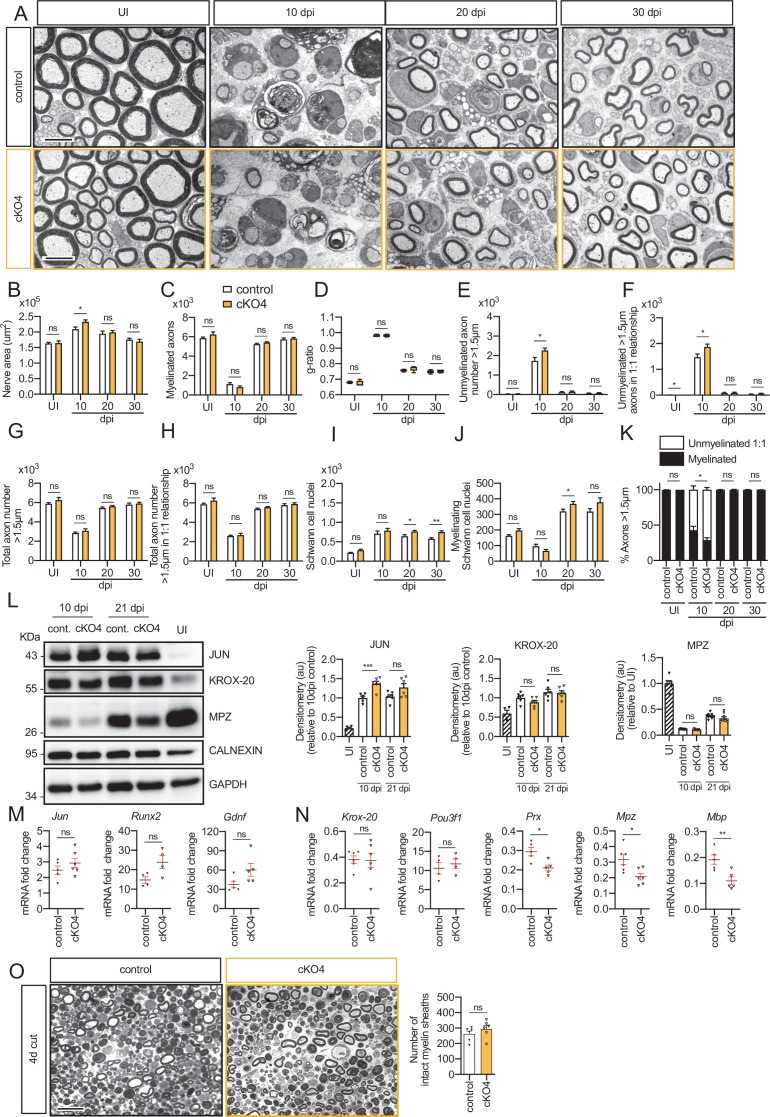
Figure 4. Remyelination is delayed in the nerves of the dKO mice.
(A) Representative transmission TEM images of P60 sciatic nerves uninjured (UI) and 10, 20, and 30 days post crush (dpi) of dKO (Mpz-Cre+/−; Hdac4flx/flx; Hdac5−/−) and the control (Mpz-Cre−/−; Hdac4flx/flx; Hdac5−/−) littermates are shown. Scale bar: 5 μm. (B) No statistically significant differences were observed in the area of the dKO nerves and control littermates (UI: p = 0.804; 10 dpi: p = 0.195; 20 dpi: p = 0.559; 30 dpi: p = 0.0594). (C) The number of myelinated axons is notably decreased at 10 dpi (388 ± 55 in the dKO versus 1.889 ± 330 in the control; p = 0.0005). (D) g ratio was increased at 10 dpi (0.989 ± 0.003 in the dKO versus 0.934 ± 0.015 in control [p = 0.002]) and at P21 (0.776 ± 0.003 in the dKO versus 0.767 ± 0.003 in control [p = 0.043]). (E) The number of unmyelinated axons in a 1:1 relationship with Schwann cells was notably increased at 10 dpi (2.969 ± 203 in the dKO versus 1.512 ± 119 in controls; p = 0.0007) and at 20 dpi (224 ± 25 in the dKO versus 88 ± 14 in controls; p = 0.0016). (F) The total number of unmyelinated axons in a 1:1 relationship with Schwann cells is increased at 10 dpi (2.148 ± 155 in the dKO versus 1.158 ± 56 in the control; p = 0.0011) at 20 dpi (175 ± 20 in the dKO versus 68 ± 12 in the control; p = 0.002) and at 30 dpi (63 ± 17 in the dKO versus 22 ± 5 in the control; p = 0.043). (G) No changes in the total axon number was found (UI: p = 0.157; 10 dpi: p = 0.910; 20 dpi: p = 0.349; 30 dpi: p = 0.666). (H) Neither in the total sorted axon number (UI: p = 0.193; 10 dpi: p = 0.169; 20 dpi: p = 0.294; 30 dpi: p = 0.682). (I) The total number of Schwann cells (counted as nuclei) was increased at 20 dpi (861 ± 34 in the dKO versus 630 ± 53 in controls; p = 0.0041). (J) In contrast, the number of myelinating Schwann cells was found decreased at 10 dpi (35 ± 8 in the dKO versus 164 ± 37 in controls; p = 0.0032). (K) The percentage of myelinated axons is decreased at 10 dpi (15.5 ± 2.3% in the dKO versus 60.4 ± 4.8% in controlps; p < 0.0001), 20 dpi (96.6 ± 0.4% in the dKO versus 98.8 ± 0.2% in controls; p = 0.0016) and, although much less, at P21 (98.9 ± 0.3% in the dKO versus 99.6 ± 0.1% in controls; p = 0.0482). For these experiment, three to six animals per genotype were used; unpaired t-test was applied for statistical analysis. (L) Expression of several negative regulators of myelination and repair Schwann cell markers is enhanced at 10 dpi in the sciatic nerves of the dKO: Jun (1.51-fold; p = 0.0056), Gdnf (1.85-fold; p = 0.0025), Bdnf (2.60-fold; p = 0.001), and Olig1 (1.60-fold; p = 0.008). (M) Expression of positive regulators and myelin genes is decreased at 10 dpi in the sciatic nerves of the dKO: Krox-20 (0.47-fold; p = 0.0068), Prx (0.45-fold; p = 0.001), Mpz (0.33-fold; p = 0.005), and Mbp (0.33-fold; p = 0.012). RT-qPCR with mouse-specific primers for the indicated genes was performed and normalized to 18S rRNA. The scatter plot, which include also the mean ± SE, shows the fold change of mRNA for each gene at 10 dpi normalized to the uninjured nerve. Five to eight mice per genotype were used. Data were analyzed with the unpaired t-test with Welch’s correlation. (N) A representative WB of protein extracts from dKO, control, KO5−/− and wild-type nerves is shown. In the quantification, JUN protein remains higher in the dKO at 10 dpi (1.72 ± 0.17-fold; p = 0.012) and tend to equalize at 21 dpi. MPZ protein was found decreased by 0.32 ± 0.02-fold at 21 dpi (p = 0.02), however we could not find changes in KROX-20 (KO5−/− mice were used to compare with the wild-type littermates). Densitometric analysis was done on seven to nine WB from the same number of mice and normalized to 10 dpi WT. Data were analyzed with the unpaired t-test (*p < 0.05; **p < 0.01; ***p < 0.001; ns: no significant). See source data file one online (graphs source data) for more details.