Fig. 2 |. Insulin signal transduction and metabolic responses in peripheral tissues and their control by mirNas.
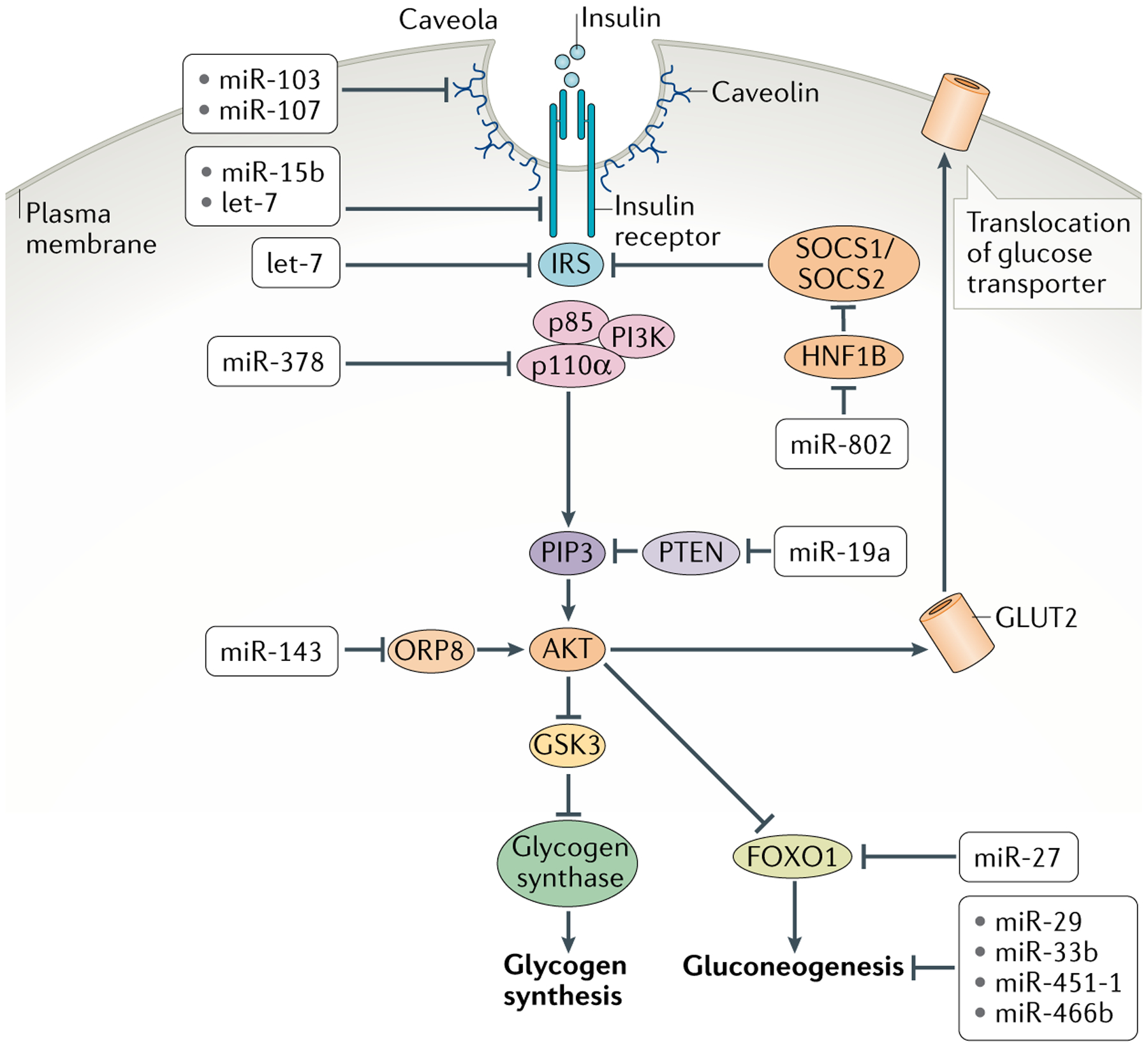
Cells bind circulating insulin via the insulin receptor, which activates insulin receptor substrate (IRS). This in turn recruits association of the regulatory p85 and catalytic p110α subunits of phosphoinositide 3-kinase (PI3K), which becomes activated. Activated PI3K catalyses the addition of phosphate groups to the 3′-OH position in the inositol ring of phosphoinositides to generate phosphatidylinositol 3,4,5-trisphosphate (PIP3). Phosphatase and tensin homologue (PTEN) converts PIP3 to hypophosphorylated isoforms. Binding of PIP3 to AKT stimulates its kinase activity, and two of its targets are glycogen synthase kinase 3 (GSK3) and the transcription factor FOXO1, both of which are repressed by phosphorylation. Phosphorylated GSK3 is blocked from inactivating glycogen synthase, resulting in synthesis of glycogen. Phosphorylated FOXO1 is blocked from transcribing genes encoding enzymes in the gluconeogenic pathway, resulting in dampening of gluconeogenesis. AKT additionally promotes glucose uptake by increasing the levels of glucose transporters at the cell membrane. Shown are the various microRNAs (miRNAs) that regulate insulin signal transduction in cells and miRNAs that regulate glycogenesis and gluconeogenesis. HNF1B, hepatocyte nuclear factor 1β; ORP8, oxysterol-binding protein-related protein 8.
