Fig. 3 |. HDL biogenesis and control by mirNas in hepatocytes.
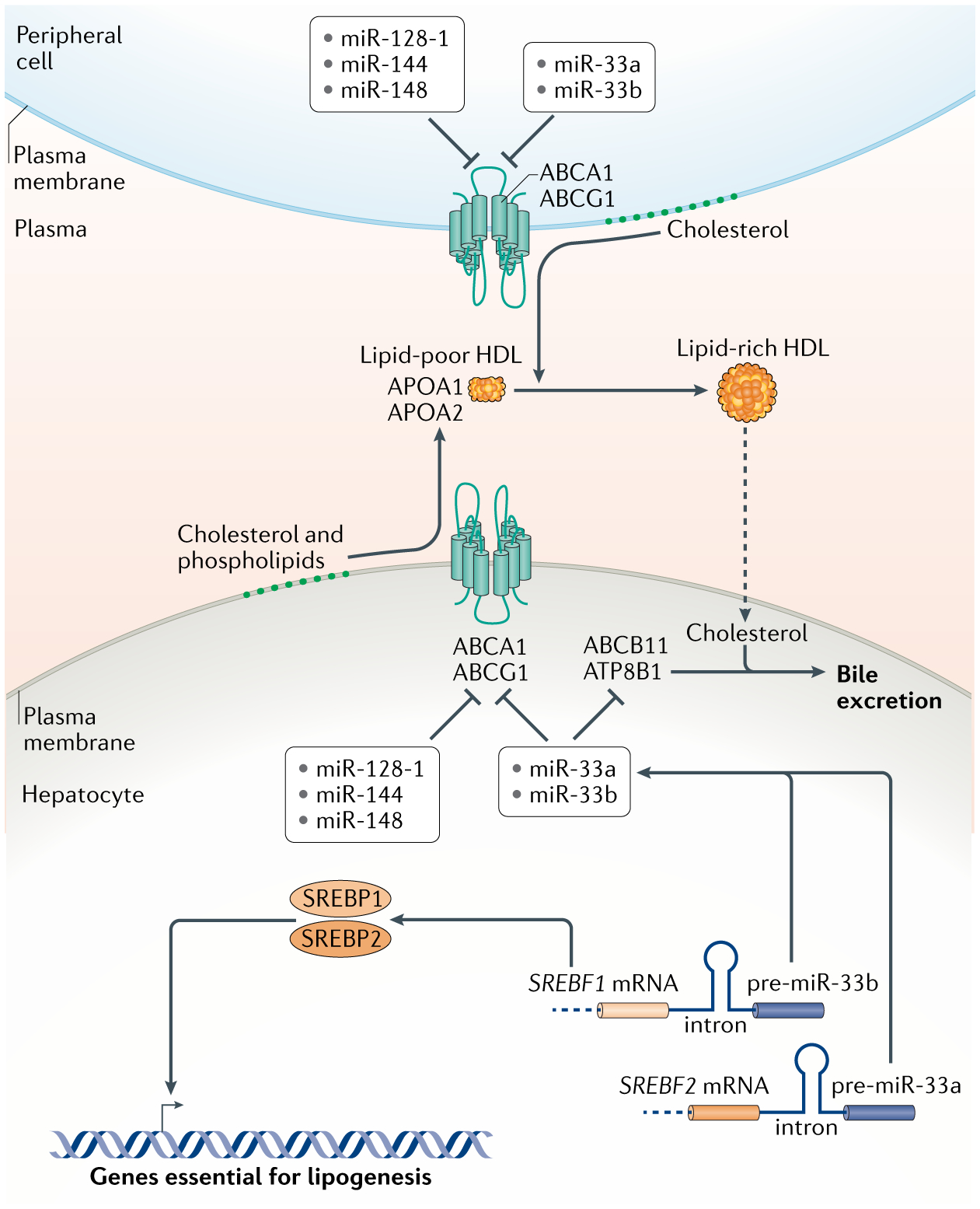
High-density lipoproteins (HDLs) are generated by the association of apolipoproteins APOA1 and APOA2 with lipids. Hepatocytes assemble extracellular lipid-poor HDL particles, which are then enriched with lipids, mostly cholesterol, derived from peripheral cells to make lipid-rich HDL. Loading of lipids into HDLs occurs via the ATP-binding cassetter transporters ABCA1 and ABCG1. The levels of these transporters are inhibited by a number of microRNAs (miRNAs) as shown. Two of these miRNAs, miR-33a and miR-33b, perform this function in concordance with two transcription factors, SREBP1 and SREBP2, which stimulate the synthesis of enzymes in lipogenic pathways. Since the precursors of miR-33a and miR-33b are located in the introns of the genes encoding SREBP2 and SREBP1 respectively, both the proteins and the miRNAs are co-expressed in tandem. miR-33a and miR-33b also inhibit expression of ABCB11 and ATP8B1, which in hepatocytes transport sterols into bile for excretion, allowing clearance of cholesterol derived from lipid-rich HDL returned to the liver after loading in the periphery. Altogether, the coordinated actions of these miRNAs and transcription factors result in accumulation of lipids within hepatocytes.
