Fig. 4 |. Control of lipid metabolism and LDL biogenesis/turnover by mirNas.
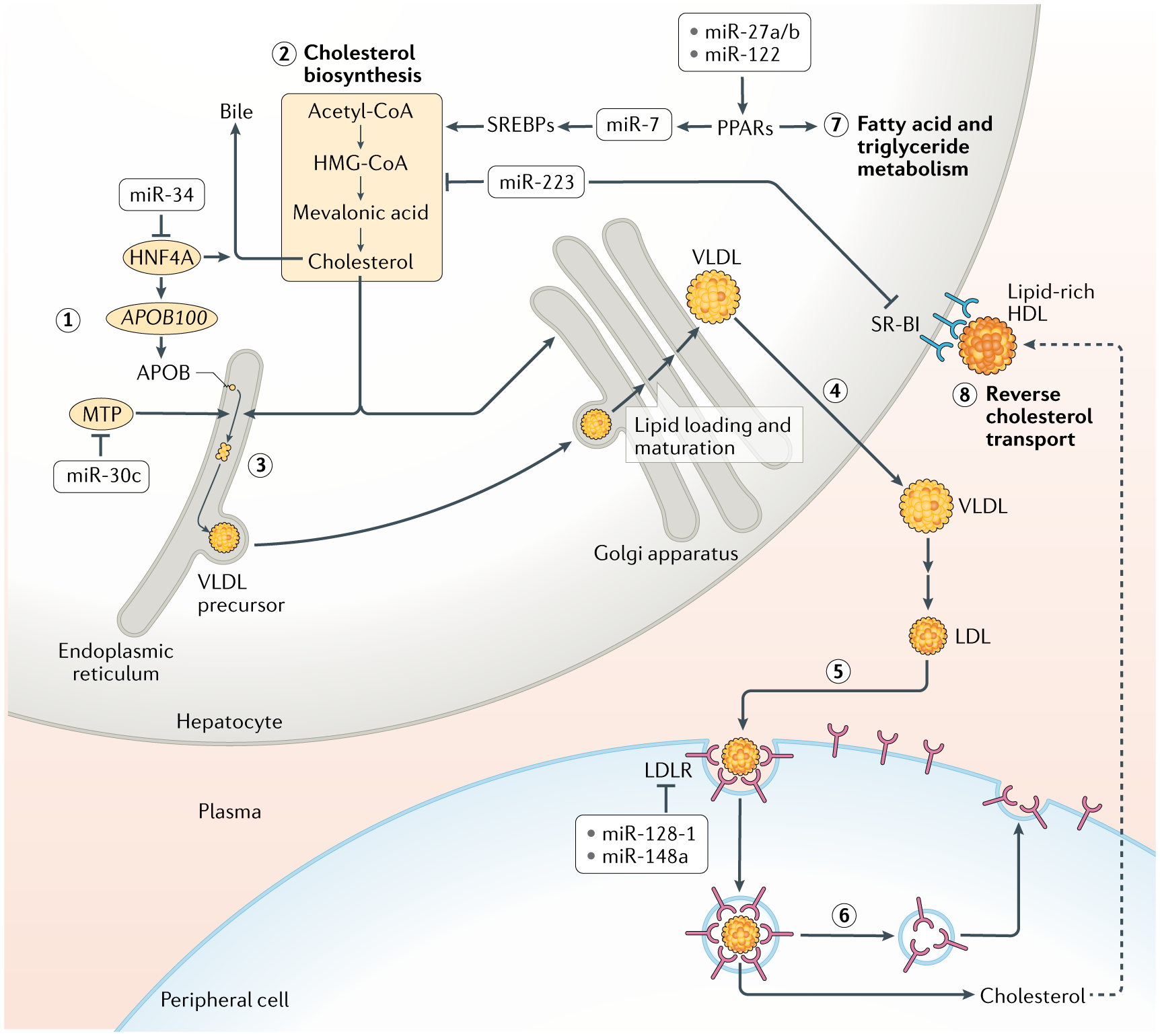
The biogenesis and turnover of low-density lipoproteins (LDL) is shown in steps 1–6. Apolipoprotein B-100 (APOB100) is synthesized and inserted into the endoplasmic reticulum of hepatocytes (1). In parallel, sterol biosynthesis converts the metabolite acetyl-CoA into cholesterol (2). Microsomal triglyceride transfer protein (MTP) transfers lipids to APOB100 to initiate assembly of very-low-density lipoprotein (VLDL) complexes in the endoplasmic reticulum (3). VLDL is shuttled to the Golgi apparatus, where it matures via lipidation, and then is secreted from hepatocytes (4). In the circulatory system, VLDL is oxidized into LDL, and this species reaches cells of the periphery. There, LDL binds to LDL receptor (LDLR) and is endocytosed (5). Sterols and other lipids are transferred out of the endosomes of peripheral cells, where they are utilized (6). The endosomes are recycled to the plasma membrane to redeposit LDLR on the surface. MicroRNAs (miRNAs) regulate virtually all steps of the LDL pathway, as indicated. Moreover, some miRNAs also regulate enzymes that control fatty acid and triglyceride metabolism in the liver, mostly by promoting these processes (7). However, miR-223 represses genes involved in cholesterol biosynthesis. In addition, miR-223 inhibits reverse transport of cholesterol from the peripheral cells back to the liver by repressing hepatocyte expression of the scavenger receptor SR-BI, which binds to cholesterol-loaded HDL and transports cholesterol into the liver (8). The net effect of the action of miR-223 is to reduce hepatic cholesterol levels. HNF4A, hepatocyte nuclear factor 4α; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; PPARs, peroxisome proliferator-activated receptors; SREBPs, sterol regulatory element-binding proteins.
