Abstract
We report the case of a 49-year-old female patient who underwent percutaneous coronary intervention of the right coronary and posterior descending arteries complicated with guidewire-induced coronary artery perforation. We successfully managed and sealed this perforation through the embolization of balloon pieces into the target vessel. (Level of Difficulty: Advanced.)
Key Words: coronary angiography, complication, percutaneous coronary intervention
Abbreviations and Acronyms: PDA, posterior descending artery
Central Illustration
History of Presentation
A 49-year-old female who had undergone primary percutaneous coronary intervention due to anterior ST-segment elevation myocardial infarction 4 weeks earlier presented with exertional angina refractory to antianginal medication. Regarding vital signs, the blood pressure was 150/90 mm Hg with a heart rate of 80 beats/min. The physical examination revealed normal body mass index and normal heart sounds without any significant physical findings. Furthermore, the routine laboratory tests were within normal limits.
Learning Objectives
-
•
To consider an alternative for the treatment of guidewire-induced distal coronary perforation when the coil and gelatin sponge embolization are not available.
-
•
To show that balloon embolization is a safe, effective, and life-saving method for the treatment of guidewire-induced distal coronary perforation.
Past Medical History
The patient had suffered from hypertension for approximately 10 years, but there were no other cardiovascular risk factors.
Investigations
The assessment of the 12-lead electrocardiogram showed a normal sinus rhythm with deep T-wave inversion in the anterior and inferior leads due to prior myocardial infarction.
An echocardiogram revealed wall motion abnormality in the anterior and septal area with a left ventricular ejection fraction of 50%. In the coronary angiogram, the prior left anterior descending artery stent was patent, although severe stenosis was detected in the mid-part of the right coronary artery and posterior descending artery (PDA) (Figures 1 and 2). This study received approval from the University of Medical Sciences Institutional Review Board.
Figure 1.
Angiogram of the Left Side
A patent left anterior descending artery stent.
Figure 2.
Coronary Plaque
(A) A severe lesion in the mid-part of the right coronary artery. (B) A severe proximal plaque in the posterior descending artery can be seen.
Management
A 6-French Judkins right catheter was advanced into the right coronary artery ostium, and 8,000 international units of heparin were administrated. A 0.014-inch guidewire (Galeo Pro) was passed through the right coronary and posterior descending arteries. Predilatation of the posterior descending artery was performed, but the 2.25 × 16 Promus Premier drug-eluting stent did not cross through the PDA lesion. The buddy wire technique was applied using a 0.014 balanced heavyweight guidewire, and the stent was eventually crossed and deployed. After stenting, we detected a guidewire-induced distal dissection without flow limitation. We decided to ignore this complication and deploy a 4 × 22 Orsiro drug-eluting stent in the mid-part of the right coronary artery. During different projections, a small wire perforation and a dissection were detected in the PDA (Figure 3). After that, low-pressure balloon tamponade was performed 3 times, each time for a duration of 10 minutes, but was unsuccessful in sealing the perforation. Autologous fat obtained from the femoral area was embolized but also failed. We then embolized clotted autologous blood with no success. An echocardiogram showed mild pericardial effusion. There was no coil or Gelfoam (gelatin sponge) available in our catheterization center. Thus, we decided to use a new method to resolve the complication. We cut a part of the 3 × 15 Apollo catheter just before the distal marker and kept this portion of the balloon (Figure 4A). Afterward, we placed this piece of the balloon on the wire and pushed it along the guidewire toward the perforation area with a new 2.75 × 15 Apollo balloon catheter (Figure 4B). Finally, the guidewire was withdrawn, and the distal balloon segment was embolized into the PDA (Figure 5). The blood leakage caused by the perforation decreased; however, the perforation was not entirely sealed. After the first embolized balloon fragment did not succeed in closing the perforation, a second distal piece of a balloon catheter was advanced and embolized in the manner described above.
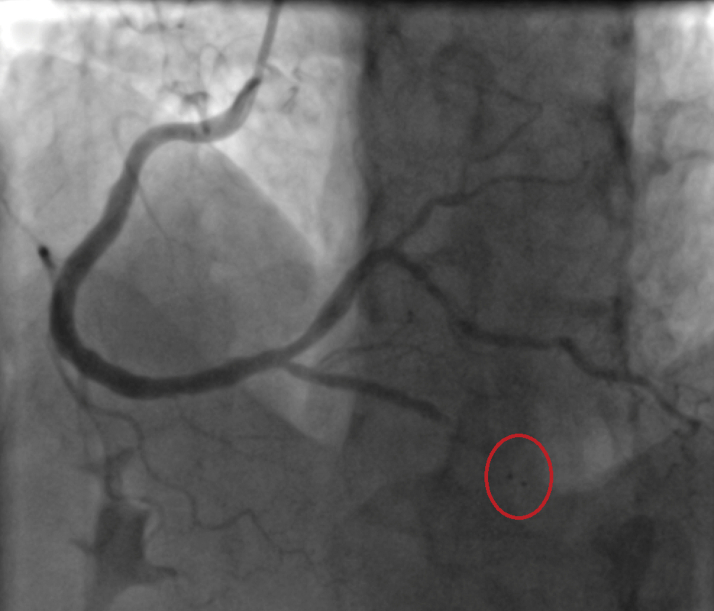
Figure 3.
Wire Perforation and Dissection
A small wire perforation and a dissection were detected in the posterior descending artery. The red circle indicates the perforation.
Figure 4.
Coronary Balloon
(A) The coronary balloon was cut from the distal part. (B) The part that was cut was placed on a 0.014-inch guidewire and was pushed by another balloon catheter.
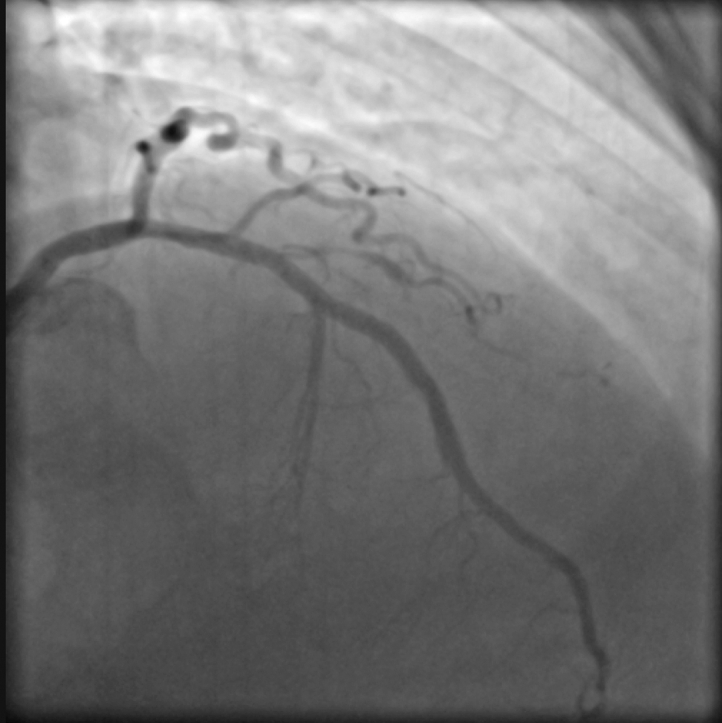
Figure 5.
Occluded Posterior Descending Artery
The posterior descending artery was completely occluded by embolized balloons. The red circle indicates the distal balloon marker.
Finally, the perforation was sealed. After sealing the perforation, we removed the guidewire and administered protamine sulfate. The follow-up echocardiogram did not show any further worsening of the effusion. The patient developed some chest pain that resolved after several hours. Laboratory assessment showed evidence of myocardial injury, with troponin I >82.00 ng/mL (normal range, <0.05 ng/mL).
The regime of aspirin and clopidogrel was continued, and the patient was discharged from the hospital after 2 days.
Discussion
Iatrogenic coronary artery perforation is a rare complication following percutaneous coronary intervention that leads to significant postprocedural morbidity and mortality (1). This event can be categorized based on the Ellis classification as type I (manifested by an extraluminal crater without extravasation), type II (featuring pericardial or myocardial blushing), and type III (where cavity spilling or contrast streaming is seen). Type I usually has a benign course (2). According to recent reports, type III perforations are associated with an in-hospital mortality rate of 44%, and two-thirds of patients may require emergency surgical intervention (3). With the recent development of novel repair techniques, the rates of mortality and emergency surgery have been reduced to 15.2% and below 5%, respectively (4). The primary approach for such perforations includes ensuring hemodynamic stability, and reversal of heparin effect by administration of Protamine sulfate. For large vessels perforation, the method of choice is to implant a covered stent and move to emergency surgery when required (3). In distal coronary perforations, several options are available for sealing the perforation such as coil embolization or the use of thrombogenic materials such as intraluminal thrombin, gelatin sponge, and polyvinyl alcohol (5). We should prioritize using these methods over other options that may be available. Moreover, using such techniques may require microcatheter or over-the-wire balloon placement (6). In these types of perforations, the vessel occlusion technique is the first choice for repairing the defect but is accompanied by the limitation of myocardial injuries (7).
In our case, because of the lack of gelatin sponge or microcoils and intraluminal thrombin in our cath lab, clot and subcutaneous fat were injected using a microcatheter, but we were not able to seal the perforation. Nonetheless, we were able to seal the perforation via balloon embolization. This technique can be an appropriate substitute for other common methods of sealing distal perforations. The performed technique has several advantages. Firstly, there is no need to repeatedly remove the guidewire and several balloon pieces can be embolized until the bleeding stops. Secondly, this method is time- and cost-efficient, and previously-used balloons can be applied. Thirdly, because of the presence of radiopaque markers on the balloon, its position in the target vessel can easily be determined. Finally, no special equipment is required. Nonetheless, this method is not flawless and has its disadvantages. One of the problems we face while applying this technique is difficulty in passing the balloon through severe calcification and tortuosity. It might also induce myocardial infarction due to vessel obstruction.
Follow-Up
The follow-up of the patient was performed in an outpatient clinic 1 week after discharge. She did not complain of chest discomfort or exertional dyspnea. Echocardiogram showed minimal pericardial effusion. She is receiving dual antiplatelet therapy and routine follow-up.
Conclusions
Overall, guidewire-induced distal coronary perforation represents a very rare although extremely lethal complication that requires immediate intervention. The treatment of choice is coil and gelatin sponge embolization; however, balloon embolization could be an alternative when the former is not available. Balloon embolization seems to be an effective method that can be used in all cath labs and requires close to no special equipment. Studies that evaluate the safety and efficacy of this technique are warranted.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Shimony A., Joseph L., Mottillo S., Eisenberg M.J. Coronary artery perforation during percutaneous coronary intervention: a systematic review and meta-analysis. Can J Cardiol. 2011;27:843–850. doi: 10.1016/j.cjca.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Hendry C., Fraser D., Eichhofer J., et al. Coronary perforation in the drug-eluting stent era: incidence, risk factors, management and outcome: the UK experience. EuroIntervention. 2012;8:79–86. doi: 10.4244/EIJV8I1A13. [DOI] [PubMed] [Google Scholar]
- 3.Al-Mukhaini M., Panduranga P., Sulaiman K., Riyami A.A., Deeb M., Riyami M.B. Coronary perforation and covered stents: an update and review. Heart Views. 2011;12:63–70. doi: 10.4103/1995-705X.86017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verevkin A., von Aspern K., Leontyev S., Lehmann S., Borger M.A., Davierwala P.M. Early and long-term outcomes in patients undergoing cardiac surgery following iatrogenic injuries during percutaneous coronary intervention. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.010940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nawale J.M., Chaurasia A.S., Borikar N.A., Nalawade D.D., Shah M.M., Shinde P.S. Single center 7 year experience of coronary artery perforation: angiographic and procedural characteristics, management and outcome. Heart Views. 2019;20:93–100. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_84_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tatli E., Vural M.G., Tokatli A., Sahinkus S. Should all coronary artery perforations be treated immediately? Arch Med Sci Atheroscler Dis. 2016;1:e103. doi: 10.5114/amsad.2016.62830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dash D. Complications of coronary intervention: abrupt closure, dissection, perforation. Heart Asia. 2013;5:61–65. doi: 10.1136/heartasia-2013-010304. [DOI] [PMC free article] [PubMed] [Google Scholar]