Abstract
The 2021 ACC/AHA/SCAI coronary artery disease revascularization guideline highlights the importance of the multidisciplinary heart team in making patient-centered, evidence-based clinical decisions for patients considered for coronary revascularization. We present 2 cases highlighting aspects of heart team decision making for complex patients with coronary artery disease. (Level of Difficulty: Intermediate.)
Key Words: clinical guideline, clinical practice guidelines, coronary artery bypass surgery, coronary artery disease, heart team, left main coronary artery disease, left ventricular dysfunction, multivessel coronary artery disease, patient-centered care, percutaneous coronary intervention
Abbreviations and Acronyms: AAA, abdominal aortic aneurysm; CABG, coronary artery bypass grafting; FDG, fluorodeoxyglucose; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; PET, positron emission tomography
Central Illustration
Case 1
The patient is a 78-year-old man presenting with complex coronary disease. Twenty years before presentation, he had thyroid cancer treated with surgical resection and radioactive iodine followed by recurrence. This was treated with radiotherapy and repeat surgical resection requiring muscle flap coverage and tracheostomy. One year ago, he had the onset of dyspnea on exertion. Symptoms progressed and became lifestyle limiting, and so he sought evaluation.
Learning Objectives
-
•
Understand the indications for heart team decision making for coronary revascularization.
-
•
Describe the composition and conduct of effective revascularization heart teams.
-
•
Understand how revascularization decisions with the heart teams should weigh risks and benefits of treatments in the context of patient preferences.
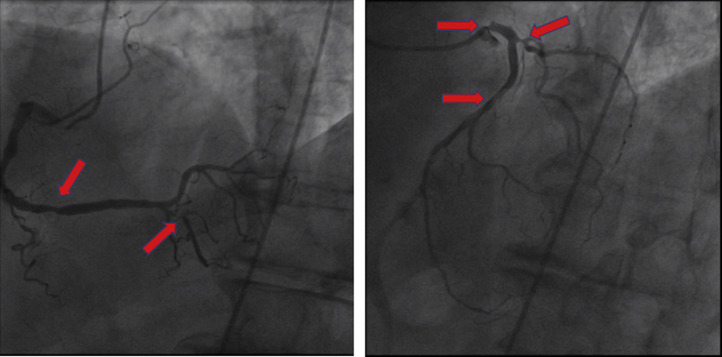
A myocardial single photon-emission computed tomography scan revealed a fixed inferoapical perfusion defect. Echocardiography demonstrated a left ventricular ejection fraction (LVEF) of 60%, and a Holter monitor evaluation reported a 12% burden of premature ventricular complexes. Because of symptoms of new dyspnea believed to be an anginal equivalent, as well as a perfusion defect on stress testing, he was referred for coronary angiography. Coronary angiography (Figure 1, Videos 1 and 2) demonstrated 90% proximal left main coronary artery stenosis with catheter damping on engagement, an eccentric 70% proximal to middle left anterior descending artery stenosis, 80% first diagonal artery stenosis, 70% ostial circumflex artery stenosis, and 90% posterior descending artery stenosis. At this point, the patient was referred for consideration of revascularization options.
Figure 1.
Coronary Angiography: Case 1
Case 1: coronary angiography demonstrating 90% proximal left main artery stenosis and 70% left anterior descending artery stenosis, 70% ostial circumflex artery stenosis, and 90% posterior descending artery stenosis (arrows).
What are the indications for heart team discussion for coronary revascularization?
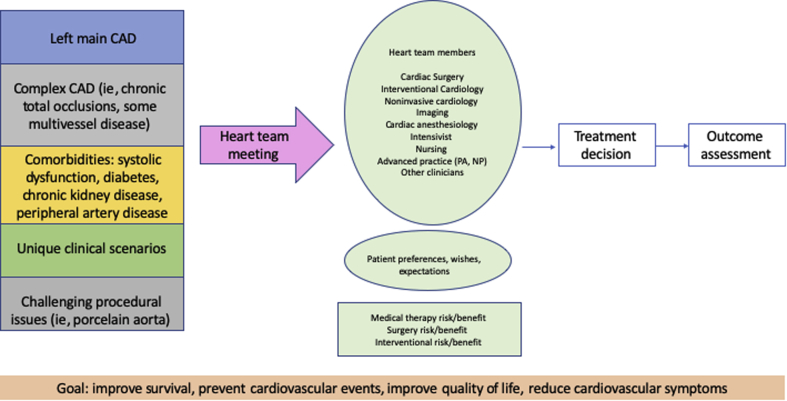
The heart team for coronary revascularization is a multidisciplinary team convened to evaluate complex patients for coronary revascularization.1 Heart team assessment is now recommended for clinical use to ensure that patient-centered, evidence-based, multidisciplinary clinical decisions are rendered. The revascularization guidelines describe several indications for heart team assessment2 (Figure 2). Indications on the basis of coronary anatomy include left main artery stenosis and multivessel coronary artery disease. Patient comorbidities that serve as considerations for heart team assessment include diabetes and systolic dysfunction. The heart team is useful in when there are barriers to the technical conduct of coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI), such as difficulties with arterial access or chest wall disease, porcelain aorta, or an anticipated hostile chest. Finally, clinicians or patients should request heart team assistance whenever there is clinical equipoise among treatment options or when other considerations could affect the success of a coronary revascularization modality.
Figure 2.
Heart Team Decision Making
Case 1: schematic depicting indications for heart team discussion for coronary revascularization, team members, clinical factors to consider, and outcomes assessment. CAD = coronary artery disease.
In this case, the patient had complex stable coronary artery disease, a significant oncologic history, and upper chest wall disease anticipated to affect the feasibility and risk of performing a sternotomy. Therefore, the heart team was convened.
For this case, the heart team first determined that revascularization was indicated. Initial studies in a previous era comparing CABG and medical therapy for left main artery disease suggested a survival advantage for CABG leading to a class I recommendation in the 2021 ACC/AHA/SCAI coronary artery disease revascularization guideline.2 There are no trials directly assessing PCI vs medical therapy for left main artery disease; however, indirect evidence suggests a survival advantage in this setting, and the 2021 ACC/AHA/SCAI coronary artery disease revascularization guidelines give a class 2a recommendation for PCI to improve survival over medical therapy for left main artery disease.2
Which clinicians should be represented on a heart team for coronary revascularization?
The revascularization heart team should include representatives from cardiac surgery, interventional cardiology, and noninvasive cardiology. Participation by nursing and advanced practice providers, perfusion, respiratory therapy, physical and occupational therapy, and cardiovascular pharmacy can be useful and is also encouraged. Other medical specialties to be considered for inclusion are critical care, anesthesiology, and imaging. Palliative care, geriatrics, oncology, and other unique specialties are included on an as-needed basis (Figure 2).
The heart team discussion for this patient included cardiology, cardiac surgery, critical care, anesthesiology, oncology, and otolaryngology, among others.
What are best practices for convening and conducting a heart team meeting for coronary revascularization?
The specific workflow for heart team meetings varies according to local resources, availability, and clinician preferences. Regular weekly meetings, virtual meetings, and emergency ad hoc discussions have all been described.3 Administrative support collating cases to be discussed, the presence of clinicians knowledgeable about cases being discussed, audiovisual equipment enabling real-time review of angiograms, and institutional support such that clinicians can attend and participate in the meeting are essential.4 The chair of the meeting should ensure that a diverse and inclusive atmosphere is created, that respectful listening is enforced, that all perspectives are heard, and that there is a defined mechanism to reach consensus for each case. There should also be consideration of how to manage instances when consensus is not reached.
How should heart teams assess the risks and benefits of the mode of coronary revascularization?
Validated risk-scoring systems, including the STS (Society of Thoracic Surgeons) score and the EuroSCORE (European System for Cardiac Operative Risk Evaluation), provide a quantitative assessment of risk for patients considered for surgical revascularization. High bleeding risk has been defined for patients considered for PCI.5 Clinical factors that are not represented in quantitative risk scores include chest wall disease, porcelain aorta and the presence of a hostile chest, the ability to comply with postprocedure therapy such as dual antiplatelet therapy, and frailty. Finally, the heart team should have a firm understanding of the patient’s preferences, goals, and wishes.6
The patient had a 1.3% STS predicted risk of mortality; however, the team believed that this calculation significantly underestimated operative risk related to sternal wound complications on the basis of previous radiation therapy to the base of neck, multiple muscle flap procedures at the superior chest, and permanent tracheostomy. Revascularization with PCI was recommended in this case.
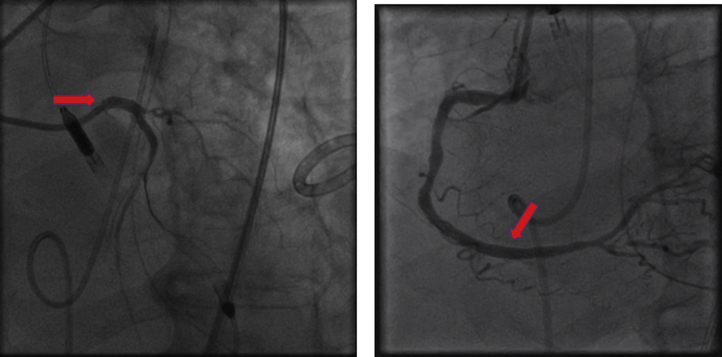
PCI of the left main and right coronary arteries with mechanical support was performed (Figure 3, Videos 3 and 4): the left main artery lesion was successfully treated with a 3.5 × 11 mm drug-eluting stent and intravascular ultrasound guidance, and the right coronary artery lesion was treated with a 3.5 × 18 mm drug-eluting stent. The left circumflex and left anterior descending artery lesions were elected to be managed medically. There were no postprocedure complications after the PCI procedures. He was discharged and was doing well at follow-up, and he was able to exercise daily with minimal symptoms.
Figure 3.
Percutaneous Coronary Intervention: Case 1
Case 1: images depicting successful left main and right coronary artery percutaneous coronary intervention with Impella assist (arrows).
Clinical perspective
This case illustrates principles of heart team review of complex patients considered for coronary revascularization. Tenets of effective heart team review include diverse specialty representation, weighing of risks and benefits, and a commitment to patient-centered care.
Case 2
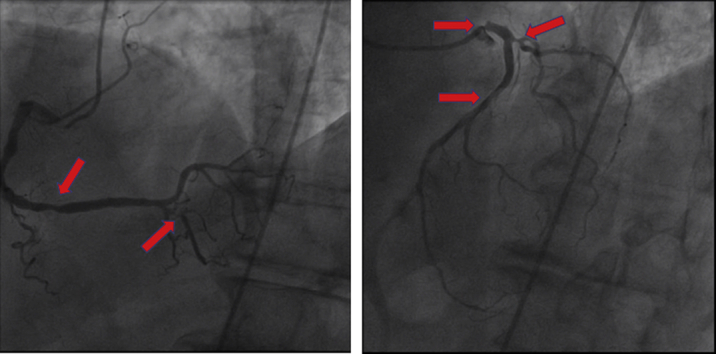
A 65-year-old man presented with urosepsis secondary to an obstructing ureteral stone. He was incidentally found to have a 6-cm abdominal aortic aneurysm (AAA) (Figure 4). In the setting of urosepsis, his troponin I level rose to peak of 2.89 ng/mL (normal <0.04 ng/mL). An echocardiogram demonstrated an LVEF of 15% with global hypokinesis (Video 5) and mild right ventricular dysfunction. Coronary angiography demonstrated a left-dominant system 90% ostial left anterior descending stenosis and 70% stenosis at the ostial circumflex (Figure 5, Video 6). He was referred to the heart team for consideration of the mode of revascularization.
Figure 4.
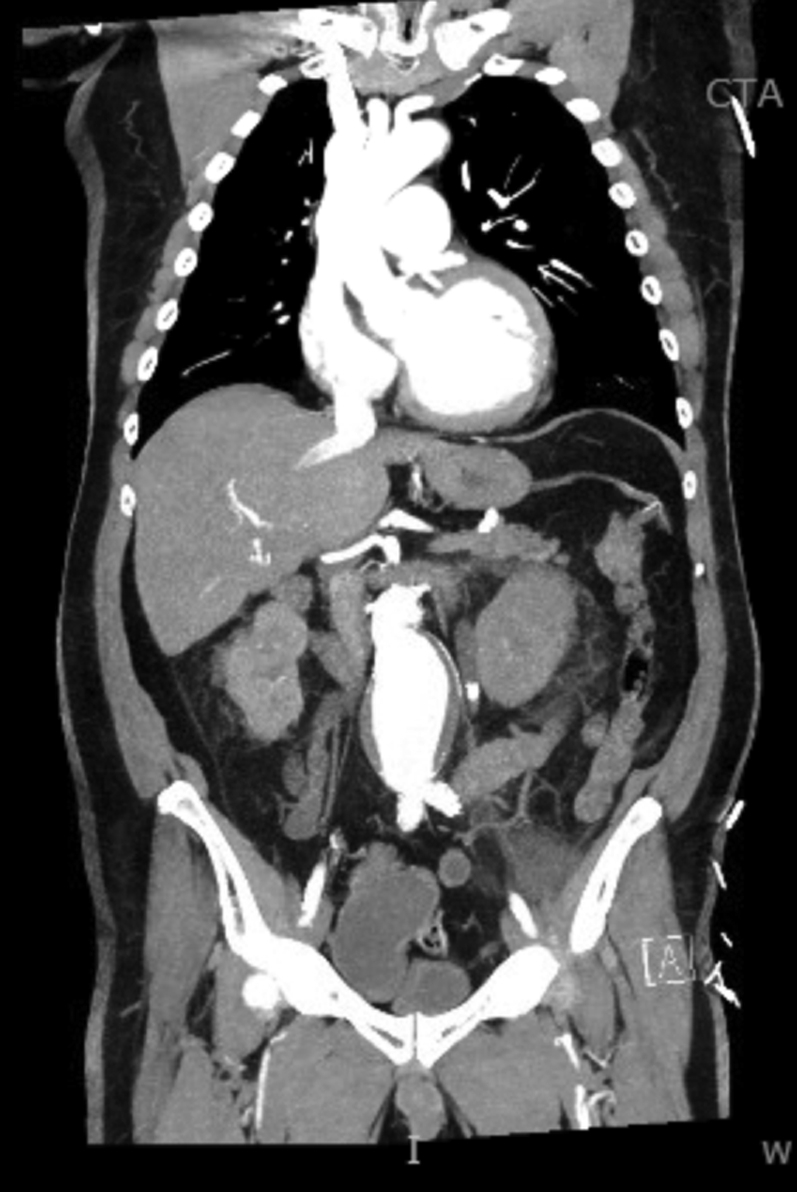
Computed Tomography Angiogram: Case 2
Case 2: Computed tomography angiography image of incidentally discovered abdominal aortic aneurysm.
Figure 5.
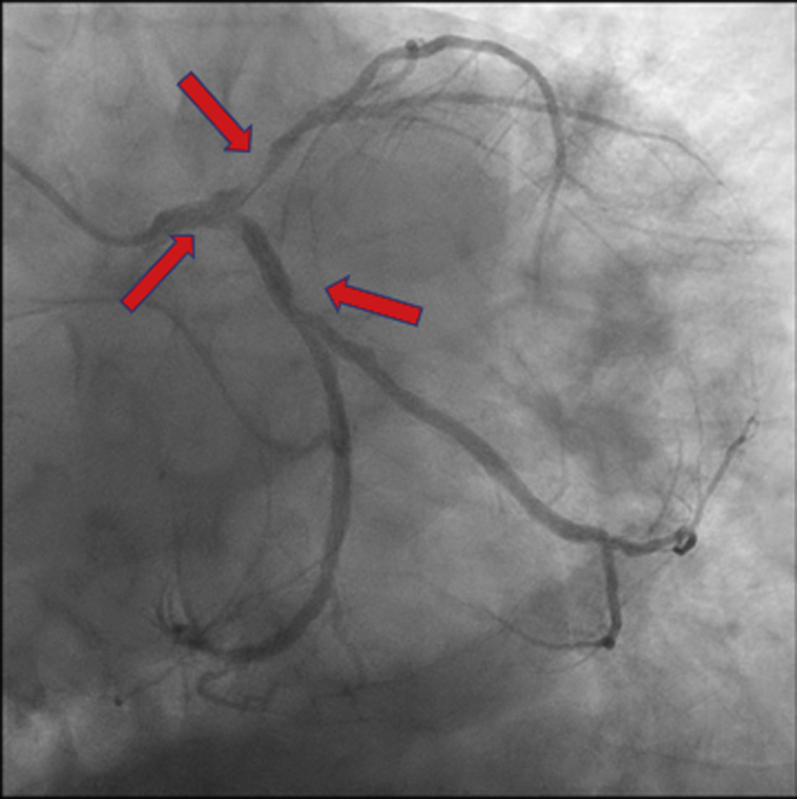
Coronary Angiography: Case 2
Case 2: coronary angiography demonstrating a left-dominant system, hazy terminal left main artery stenosis, 90% ostial left anterior descending artery stenosis, and 70% stenosis at the ostial circumflex artery (arrows).
Given the left main artery disease, systolic dysfunction, need for future AAA repair, the heart team recommended CABG. The intent of revascularization in this case was to improve survival. The pivotal STICH (Surgical Treatment for Ischemic Heart Failure) trial did not show a survival benefit to CABG over medical therapy in systolic dysfunction at 5 years, but a survival benefit emerged at the 10-year follow-up.7 The 2021 ACC/AHA/SCAI coronary artery disease revascularization guidelines give a class I recommendation for CABG to improve survival over medical therapy in this setting.2 This issue was discussed with the patient, and CABG was advised. The patient relocated to another geographic region, and so CABG was planned at a different institution.
When should noncardiac clinicians be included in heart team deliberations before coronary revascularization?
Clinicians from different specialties should be included in the heart team discussion when the coronary revascularization procedure affects the timing and conduct of other necessary specialty care. In this case, vascular surgery should be involved in discussions regarding the timing of revascularization and AAA repair; it was expected and confirmed that revascularization of left main coronary artery disease should occur before AAA repair.
The patient went on to have fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging to assess myocardial viability before the heart team convened. The LVEF on the nuclear study was calculated at 16%.
How should heart teams integrate and interpret advanced imaging studies of myocardial viability?
The use of myocardial viability studies to guide coronary revascularization is controversial.8 In many practices, viability is not always used as a sole criterion for or against revascularization; rather, it is used selectively as a single piece of data within the individual clinical context. Heart teams should decide a priori how to integrate viability assessment into their decisions, decide which patients should have viability testing, and should ascertain what testing should be performed. Including imaging experts in patient discussions is critical.
The FDG-PET study demonstrated preserved FDG activity of the distal anterior and anterolateral wall corresponding to an area of reduced perfusion suggesting viability of this territory, whereas there was an area of the apical lateral and inferior myocardium with no FDG update and reduced perfusion consistent with scar.
What considerations should heart teams discuss beyond the decision, modality, and timing of revascularization?
Heart teams can have a clinical impact beyond the decision and modality of revascularization. For those patients undergoing CABG, the specific conduits can be discussed, including consideration of multiple arterial grafts. This decision is affected by comorbidities such as diabetes and obesity, as well as whether the radial artery has been accessed for previous coronary angiography. For those patients undergoing PCI or medical therapy, the choice of stent and the anticipated duration of antiplatelet therapy can be discussed, as well as contingency plans should bleeding complications occur.
Clinical perspective
This case illustrates heart team decision making that is informed by noncardiac comorbidities and involving additional clinical specialties. This case also highlights how heart teams can consider integrating data such as myocardial viability studies and how heart teams can add clinical value beyond the revascularization decision alone.
Funding Support and Author Disclosures
Dr Metkus has reported salary support from the National Institutes of Health (NIH)–funded Institutional Career Development Core at Johns Hopkins (project No. 5KL2TR003099-02); has reported consulting unrelated to this subject matter for TelaDoc/BestDoctors Inc; has developed of educational materials for Oakstone/EBIX; and has received royalties for a textbook publication for McGraw-Hill publishing unrelated to this subject matter. Dr Cohen has reported consulting for Abiomed, Medtronic, Terumo Medical, and Merit Medical. Dr Fremes has reported being nominated principal investigator of the Canadian Institutes of Health Research (CIHR)–funded ROMA trial; co-investigator of the NIH-funded ROMA:QofL and ROMA:Cog trials; is co-investigator of the CIHR-funded VISION ECG substudy and the DEPOSITION trial; has reported serving on the data safety monitoring board of Polypid; and has reported being a local principal investigator of studies funded by Medtronic and Boston Scientific. Dr Mehran has reported institutional research grants from Abbott, Abiomed, Applied Therapeutics, Arena, AstraZeneca, Bayer, Biosensors, Boston Scientific, CardiaWave, CellAegis, CERC, Chiesi, Concept Medical, CSL Behring, DSI, Insel Gruppe AG, Medtronic, OrbusNeich, Philips, Transverse Medical, and Zoll; has received personal fees from ACC, Boston Scientific, California Institute for Regenerative Medicine (CIRM), Cine-Med Research, Janssen, WebMD, and the Society for Cardiovascular Angiography and Interventions; has reported consulting fees paid to the institution from Abbott, Abiomed, AM-Pharma, Alleviant Medical, Bayer, CardiaWave, CeloNova, Chiesi, Concept Medical, CSL Behring, DSI, Duke University, Idorsia Pharmaceuticals, Medtronic, Novartis, Philips; has reported <1% equity in Applied Therapeutics, Elixir Medical, STEL, and CONTROLRAD (spouse); has reported memberships on the Scientific Advisory Board for the American Medical Association and Biosensors (spouse); and reported being on the Faculty of the Cardiovascular Research Foundation (CRF) (no fee). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Coronary Angiography Demonstrating Left Main and Multivessel Coronary Disease
Coronary Angiography Demonstrating Left Main and Multivessel Coronary Disease
Images Depicting Successful Left Main and Right Coronary PCI With Impella (Abiomed) Assist
Images Depicting Successful Left Main and Right Coronary PCI With Impella Assist
Echocardiogram Demonstrating Severe Systolic Dysfunction and Regional Wall Motion Abnormalities
Coronary Angiography Demonstrating Left Dominant System and Complex Left Main Coronary Disease
References
- 1.Yeoh J., MacCarthy P. Is it time to refresh the heart team? New paradigms for shared decision making. Heart. 2021;107:674–681. doi: 10.1136/heartjnl-2020-316588. [DOI] [PubMed] [Google Scholar]
- 2.Lawton J.S., Tamis-Holland J.E., Bangalore S., et al. 2021 ACC/AHA/AATS/STS/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21–e129. doi: 10.1016/j.jacc.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Sadeghi A.H., Wahadat A.R., Dereci A., et al. Remote multidisciplinary heart team meetings in immersive virtual reality: a first experience during the COVID-19 pandemic. BMJ Innov. 2021;7:311–315. doi: 10.1136/bmjinnov-2021-000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luckraz H., Norell M., Buch M., James R., Cooper G. Structure and functioning of a multidisciplinary ‘heart team’ for patients with coronary artery disease: rationale and recommendations from a joint BCS/BCIS/SCTS working group. Eur J Cardiothorac Surg. 2015;48:524–529. doi: 10.1093/ejcts/ezv083. [DOI] [PubMed] [Google Scholar]
- 5.Urban P., Mehran R., Colleran R., et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Eur Heart J. 2019;40:2632–2653. doi: 10.1093/eurheartj/ehz372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vahanian A., Urena M. Incorporating patients' preferences into the heart team equation. EuroIntervention. 2020;16:784–786. doi: 10.4244/EIJV16I10A146. [DOI] [PubMed] [Google Scholar]
- 7.Velazquez E.J., Lee K.L., Jones R.H., et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374:1511–1520. doi: 10.1056/NEJMoa1602001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia M.J., Kwong R.Y., Scherrer-Crosbie M., et al. State of the art: imaging for myocardial viability: a scientific statement from the American Heart Association. Circ Cardiovasc Imaging. 2020;13 doi: 10.1161/HCI.0000000000000053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coronary Angiography Demonstrating Left Main and Multivessel Coronary Disease
Coronary Angiography Demonstrating Left Main and Multivessel Coronary Disease
Images Depicting Successful Left Main and Right Coronary PCI With Impella (Abiomed) Assist
Images Depicting Successful Left Main and Right Coronary PCI With Impella Assist
Echocardiogram Demonstrating Severe Systolic Dysfunction and Regional Wall Motion Abnormalities
Coronary Angiography Demonstrating Left Dominant System and Complex Left Main Coronary Disease