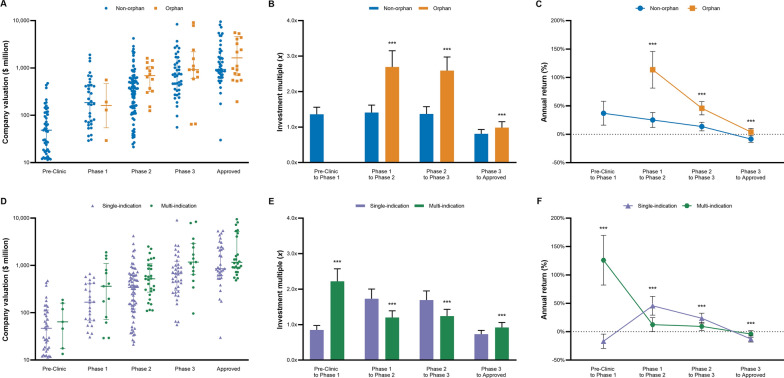
Fig. 1.
Company valuation, investment multiples, and annual returns by lead drug’s FDA orphan designation status and number of indications. Graphs in the first row compare the valuation (A), investment multiples (B), and annual returns (C) for companies with orphan- and non-orphan-designated lead drugs by development stage. Graphs in the second row compare the valuation (D), investment multiples (E), and returns (F) for companies with multi-indication and single-indication lead drugs by development stage. Valuation data from our sample of 311 Biopharma acquisitions (2005–2020) were inflation adjusted to 2020 values and combined with previously published success rates and development periods to calculate multiples and returns [11, 16]. No valuation data exist for the Pre-Clinic orphan category given that the FDA only issues the orphan designation status after IND approval. P values calculated based on ANOVA with Dunnett’s test: *p < 0.05, **p < 0.01, ***p < 0.001. FDA US Food and Drug Administration