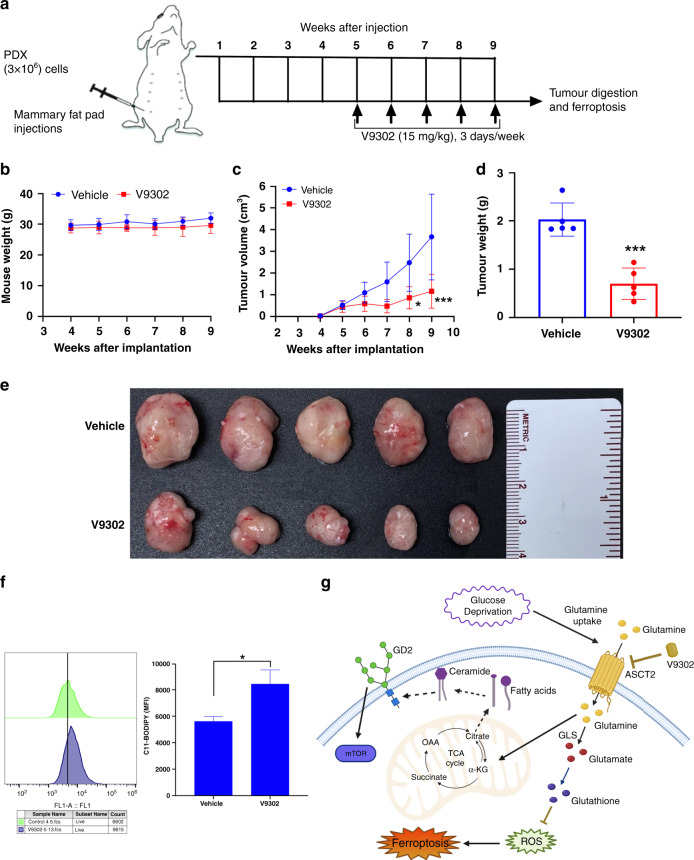
Fig. 6. Inhibition of glutamine metabolism reduces in vivo tumorigenesis.
a Scheme of the treatment plan of mice injected with PDX cells. After the palpable tumours formed at week 5, mice were treated alone or in combination with V9302 (15 mg/kg) for 3 days per week. b Changes in the mouse weight from each group was monitored on weekly basis until the end of the study. c–e PDX cells were implanted (3 × 106 per mouse) into the mammary fat pads of NSG mice (n = 20, 5 mice per group). Once palpable tumours were formed, mice were randomly grouped into V9302 and vehicle control (2% DMSO in PBS) groups; V9302 treatment (15 mg/kg) was given thrice a week until the end of the study. After the tumour diameters reached 2 cm, mice were euthanized for tumour visualisation; shown are the tumours at 9 weeks after the implantation and tumour weights were measured. One-way ANOVA with Bonferroni’s multiple comparison test was performed for tumour volumes, *p < 0.05 and ***p < 0.001. An unpaired t test was performed for the tumour weights, ***p < 0.001. f In vivo ferroptosis on the cells isolated from the PDX tumours obtained from mice treated with vehicle or V9302. Lipid peroxide levels were measured by C11-BODIPY staining using flow cytometry. An unpaired t test was performed between untreated and V9302-treated tumour cells. *p = 0.033. g Summarised illustration of metabolic stress-inducing glutamine uptake that may contribute to GD2 biosynthesis through TCA cycle and fatty acid synthesis (dotted arrows). Glutamine is required for glutathione synthesis and homeostasis of ROS. However, inhibition of ASCT2 by V9302 not only downregulate GD2 expression but also inhibits glutathione production and induces ferroptosis.