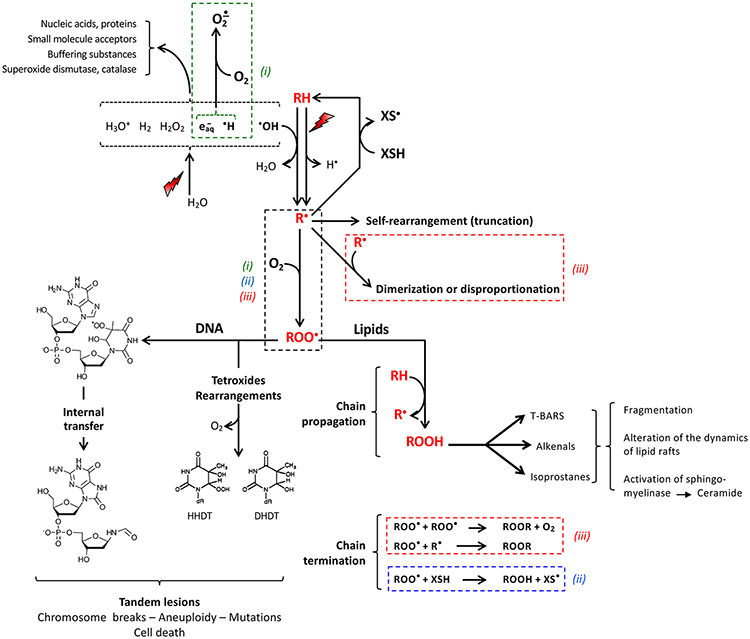
Figure 2.
General scheme summarizing the oxygen-dependent reactions leading to radiation-induced degradation of nucleic acid and lipids. The reaction starts with hydrogen atom abstraction from the carbon atom of a substrate RH either by direct energy transfer or indirectly by reaction with the •OH radical released from water radiolysis (Figure 1), approximated to ca. 30% and 70%, respectively98,99. Water-derived radicals are trapped by the large amount of acceptors present in cells, including oxygen generating the superoxide anion by reaction with e−aq and H• (upper left). The alkyl or allyl radical R• evolves through scavenging by hydrogen donors (XSH) including ascorbate, α-tocopherol or non-protein thiols; by internal reorganization; by bimolecular recombination; or by reaction with oxygen in the course of a diffusion-controlled reaction yielding the peroxyl radical ROO•.
In nucleic acids, peroxyl radicals may evolve following either one of two paythways. Firstly, by self-rearrangement or disproportionation via tetroxide intermediates releasing O2 and a wealth of products. HHDT (6-hydroperoxy- 5-hydroxy-5,6-dihydrothymine) and DHDT (5,6-dihydro-5,6-dihydroxythymine) are shown as examples. Secondly, via generation of tandem lesions by reaction with vicinal nucleobases. The peroxyl radical formed at C-5 of thymidine and the end-product of its reaction with vicinal deoxyguanosine100 is shown as an example.
In unsaturated lipids, ROO• radicals initiate a chain reaction starting with oxidation of another molecule of lipid RH with release of a new molecule of R•. Lipid peroxides decompose into by-products including alkenals, isoprostanes and thiobarbiturtic acid reactive substances (TBARS). Termination of the chain involves radical–radical annihilation, with release of O2 from recombination of ROO• with itself, or reduction by thiol-like compounds.
The boxes (dotted line) underscore the main parts of the reaction network on which the hypotheses (i), (ii) and (iii) in Introduction are based. The TOD model (i) assumes that oxygen depletion occurs through reaction with e−aq and H• and upon generation of ROO•. Model (ii) stems from a competition between the bimolecular recombination of R• and the reaction R• + O2 on the one hand, and chain termination by radical recombination involving peroxyl radicals. The cell-specific differences in the ability to detoxify from reactive oxygen species underlying hypothesis (ii) assumes that normal tissues are able to remove organic peroxyl radicals and peroxides more effectively than tumor tissues.