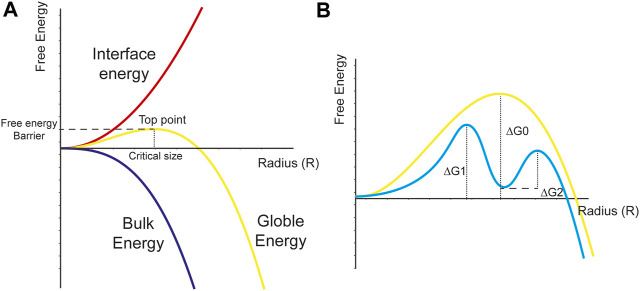
FIGURE 1.
Diagrammatic sketch for classical nucleation theory.(A) The bulk energy is proportional to the cube of nuclei radius. Whereas the interface energy is proportional to the square of nuclei radius. The top point of global free energy is called the free-energy barrier, and the nuclei radius which could make the global system overcome the free-energy barrier is defined as the critical size. (B) The solute could firstly overcome the free energy barrier ( ) to achieve a metastable state. Solute in the dense droplets could then overcome the free energy barrier ( ) to accomplish the nucleation process.