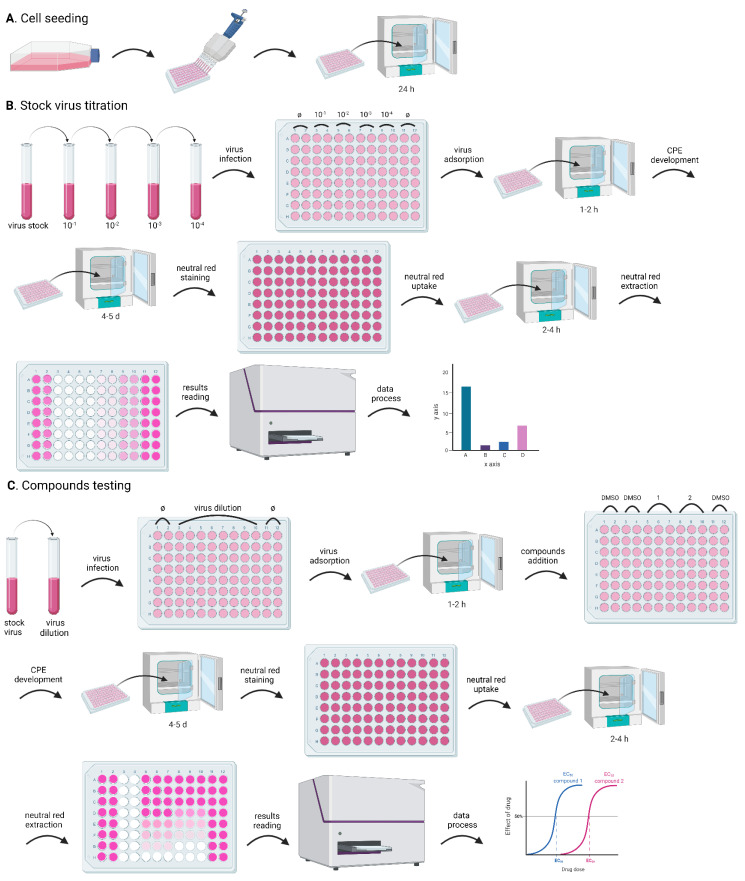
Figure 1. Flowchart for the CPE assay.
The whole process includes: cell seeding, to prepare cells in a 96-well plate for the experiments; stock virus titration, to optimize the assay conditions; and compound testing, for the evaluation of the antiviral activity of test compounds. Both stock virus titration and compound testing comprise the following steps: dilute the stock virus to obtain the desired MOI; infect the cells in the 96-well plate with a small volume (100 µL) of the diluted virus; incubate the 96-well plate in the incubator for 1-2 h, to facilitate viral attachment (virus adsorption); add the test compounds (only for the compound testing experiment); incubate the 96-well plate in the incubator to develop CPE; stain the cells with neutral red; extract neutral red from the cells; quantify neutral red by measuring absorbance; analyze data. A. Preparation of cells in the 96-well plate for the CPE assay; B. Major steps of stock virus titration in the CPE assay; C. Assessment of antiviral activity of test compounds in the CPE assay.