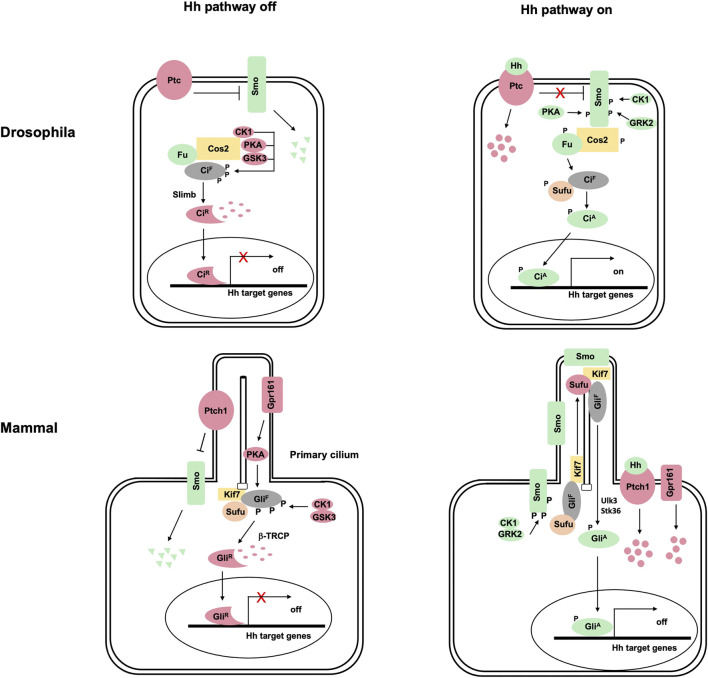
FIGURE 1.
The hedgehog signaling transduction in Drosophila and vertebrates. (A) In Drosophila, the 12-span transmembrane protein Ptc represses the GPCR-family protein Smo in the absence of Hh ligand, leading to the degradation of Smo by the ubiquitylation pathway. Full-length Ci (CiF) forms a complex with the kinesin-like protein Cos2 and the Ser/Thr kinase Fu, which recruits PKA, GSK3, and CK1 to phosphorylate Ci. Phosphorylation of CiF targets it for Slimb-mediated ubiquitination, followed by proteasome-mediated proteolysis to generate CiR. Binding of Hh to Ptc releases its inhibition of Smo, allowing Smo to be phosphorylated by PKA, CK1 and GRK2. Activated Smo not only blocks CiF phosphorylation by PKA/GSK3/CK1 and thus the production of CiR but also converts CiF into the activator form (CiA) through activating the Fu kinase, which directly phosphorylates CiF to release its inhibition by Sufu. (B) Vertebrate Hh signal transduction depends on primary cilia. In the absence of Hh, both Ptch1 and Gpr161 (a GPCR coupled to G⍺s) are localized in primary cilia where Ptch1 prevents Smo ciliary localization and Gpr161 activates PKA. Full-length Gli (GliF; mainly Gli3 and Gli2) is phosphorylated by PKA, GSK3, and CK1, which targets it for β-TRCP-mediated ubiquitination, followed by proteasome-mediated proteolysis to generate GliR. Binding of Hh to Ptch1 inhibits its activity and promotes its ciliary exit, allowing Smo to be phosphorylated by CK1 and GRK2 and accumulated in primary cilia. Activated Smo inhibits Ci phosphorylation by PKA and the production of GliR in part by promoting Gpr161 ciliary exit. In addition, activated Smo converts GliF into GliA at least in part through Ulk3 and STK36, which phosphorylate GliF to attenuate the inhibition by Sufu.