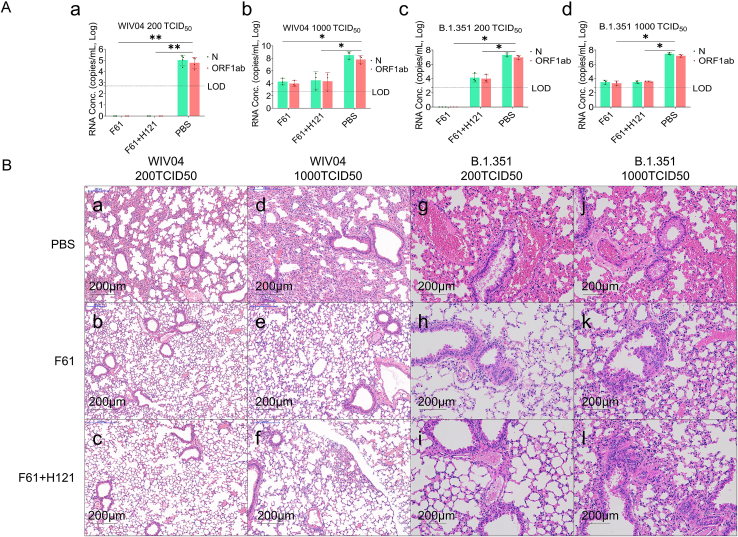
Fig. 3.
Antibodies provided prophylactic protection against alterations in lung pathology following inoculation with the wild strain and B.1.351 variant of SARS-CoV-2. Mice were pretreated with 20 mg/kg F61 or F61+H121 cocktail via intranasal route 2 h before challenge with WIV04 or the beta variant. The same volume of PBS was administered via intranasal route in the negative control. Lungs were harvested from each group (n = 3). A Viral RNA levels in the lungs were measured by qRT-PCR (copies/mL). (A-a) 200 TCID50 WIV04, (A-b) 1000 TCID50 WIV04, (A-c) 200 TCID50 beta variant, and (A-d) 1000 TCID50 beta variant. B Histopathological analyses of mice pretreated with or without monoclonal antibodies and infected with SARS-CoV-2 strains. Lungs were fixed for sectioning before staining with hematoxylin and eosin. (B-a–B-l) Lung sections from mice treated PBS, F61, and F61/H121 cocktail before challenge with 200 TCID50 WIV04 (B-a–B-c), 1000 TCID50 WIV04 (B-d–B-f), 200 TCID50 beta variant (B-g–B-i), or 1000 TCID50 beta variant (B-j–B-l). All data points are shown, along with medians. ns, P > 0.05; ∗, P < 0.05; ∗∗, P < 0.0001, as determined by Student's t-test. Limit of detection (LOD), 500 copies/mL. Scale bars, 200 μm.