Figure 1. Prospects for single-cell proteomics by single-molecule counting.
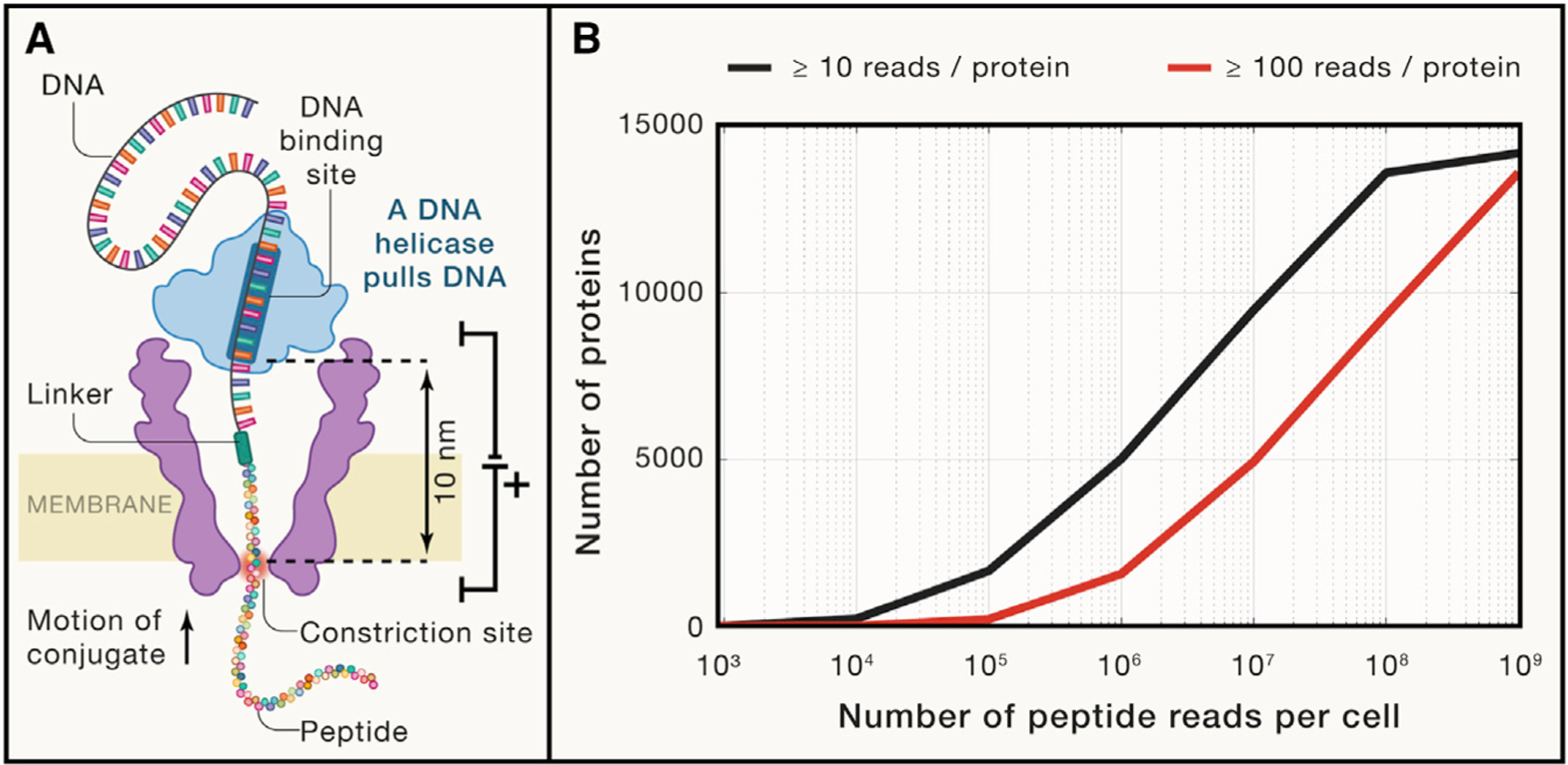
(A) A diagram of a nuclear pore used by Brinkerhoff et al. (2021) to fingerprint peptide variants. The pore is made of the mutant porin A, a channel-forming protein originally derived from Mycobacterium smegmatis. Its channel is about 1nm in diameter and is flanked by regions of larger diameter. The peptides are pulled through the pore by the attached DNA molecule pulled by a DNA helicase. As the peptide passes through the constriction zone (marked in red), the current blockades provide information for the amino acid sequence.
(B) An estimated number of proteins detected per single HeLa cell as a function of the number of peptide reads. The estimation sampled proteins with probability proportional to their abundance in HeLa cells (Bekker-Jensen et al., 2017). Peptide-specific biases and unmappable peptides were not considered by this estimation; such effects will increase the required number of reads.
