Fig. 1 |. Development of SM-derived CPT liposomal nanovesicles (camptothesomes).
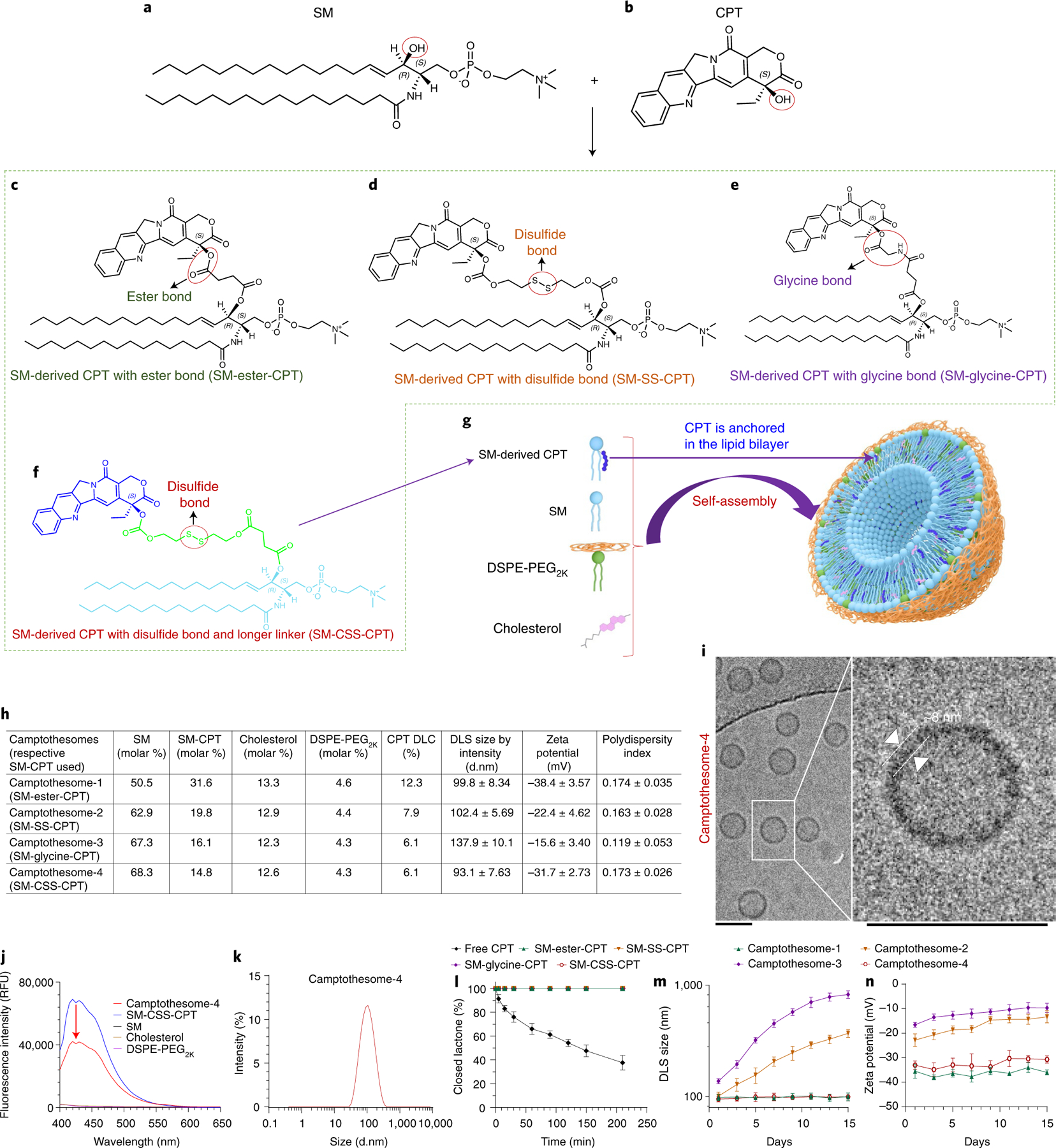
a,b, Chemical structures of SM (a) and CPT (b). c–f, Conjugation of SM and CPT resulted in SM-derived CPTs with either an ester bond (SM-ester-CPT) (c), a disulfide linkage (SM-SS-CPT) (d), a glycine bond (SM-glycine-CPT) (e), or a disulfide linkage and a longer linker (SM-CSS-CPT) (f). g, Schematic depicting the self-assembly of SM-CPT into a camptothesome. h, A table delineating the physicochemical characterizations of the four camptothesomes. DLS, dynamic light scattering; d.nm, diameter values in nanometres. i, cryo-EM for camptothesome-4. Scale bars, 100 nm. j, The fluorescence intensities (relative fluorescence units, RFU) for camptothesome-4, SM-CSS-CPT, SM, cholesterol and DSPE-PEG2K in methanol at equivalent concentrations. The significant fluorescence quenching for SM-CSS-CPT after self-assembly into camptothesome-4 demonstrates that there are strong π–π stacking interactions among SM-CSS-CPT molecules since CPT contains several aromatic rings. k, Representative DLS size distribution by intensity for camptothesome-4. For i–k, three independent experiments were performed to confirm the results. l, Percent closed lactone in PBS (pH 7.4) as a function of time for free CPT and the four different SM-CPT conjugates. SM-conjugated CPTs strongly prevented the lactone form from being converted into the inactive carboxylate form, enhancing CPT stability. m,n, Monitoring of the DLS size by intensity (m) and zeta potential (n) for each of the four different camptothesomes over time in 5% dextrose at 4 °C. Data in h (right portion) and l–n are expressed as mean ± s.d. (n = 3 independent experiments).
