Figure 1.
Purified MeCP2 forms liquid-like droplets in physiologically crowding environments.
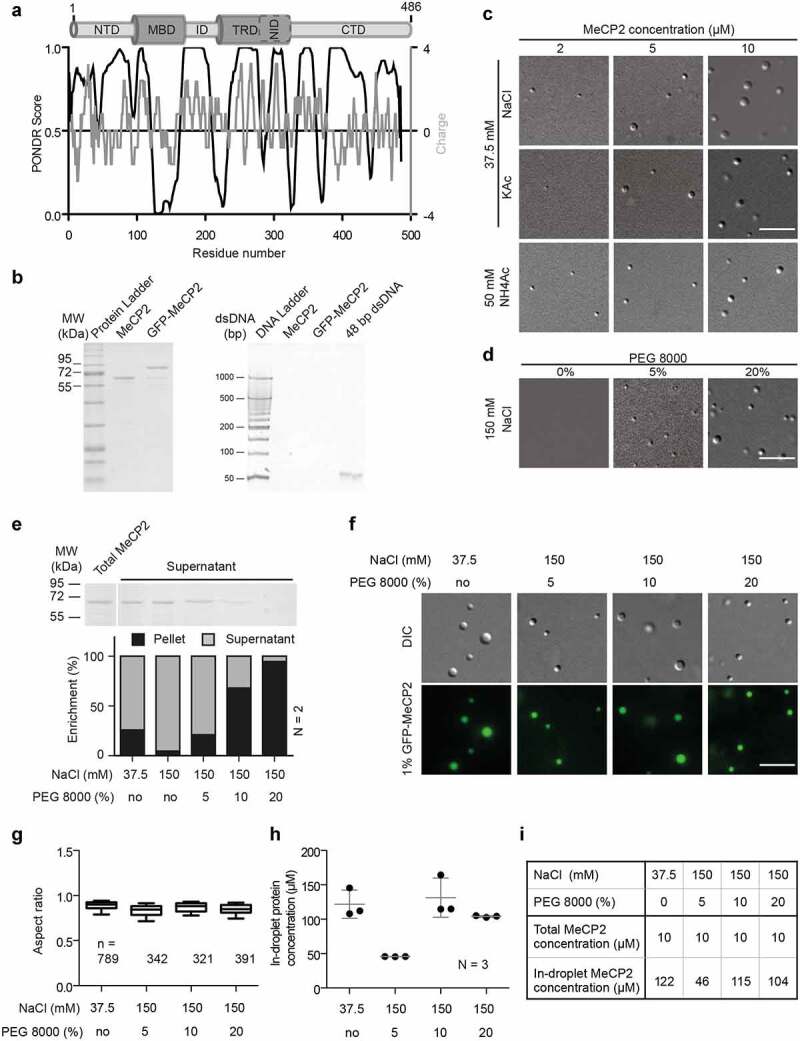
(A) Analysis of human MeCP2 protein sequence. Top: Schematic overview of human MeCP2 structure. NTD: N-terminal domain; MBD: methyl binding domain; ID: intervening domain; NID: N-CoR interacting domain; CTD: C-terminal domain; TRD: transcriptional repression domain. Bottom black line: PONDR prediction (http://www.pondr.com/) of MeCP2 ordered/disordered regions, >0.5 is considered disordered. Bottom gray line: protein charge, >0 means positively charged (https://www.bioinformatics.nl/cgi-bin/emboss/charge). The Isoelectric point (PI) of MeCP2 is predicted 10.56 using INNOVAGEN (https://pepcalc.com/). Amino acid labeling is according to human MeCP2 isoform 1.(B) Validation of MeCP2 purity. The MeCP2 and GFP-MeCP2 proteins were expressed in bacteria by IPTG induction, purified using chitin beads and eluted by DTT. The final protein concentrations were measured by Pierce™ 660 nm Protein Assay Reagent. 2 µg and 10 µg purified protein were then used for SDS polyacrylamide gel electrophoresis and tris borate EDTA polyacrylamide gel electrophoresis, respectively. Left: SDS polyacrylamide gel electrophoresis of purified human MeCP2 and GFP-MeCP2 followed by Coomassie staining. 2 µg each lane. Right: tris borate EDTA polyacrylamide gel electrophoresis of purified human MeCP2 and GFP-MeCP2 followed by ethidium bromide (EtBr) staining. 10 µg each lane. ~140 ng 42 bp DNA was used as positive control.(C) DIC images of MeCP2 phase separated droplets. The in vitro phase separation assay performed at different protein concentrations in buffers containing low concentrations of monovalent cations (37.5 mM NaCl, KCl and NH4Ac). The mixtures were transferred to chambers made of double-sided tapes and sealed with coverslips 45 min after incubation at room temperature. The droplets were observed using a Nikon Eclipse TiE2 microscope equipped with differential interference contrast (DIC) microscopy. Scale bars = 10 µm.(D) DIC images of MeCP2 phase separated droplets in the presence of crowder, PEG 8000. The in vitro phase separation was done by incubating at room temperature for 45 min and the droplets were observed using the Nikon Eclipse TiE2 microscope equipped with differential interference contrast microscopy. NaCl: 150 mM, MeCP2: 10 µM. Scale bars = 10 µm.(E) Quantification of MeCP2 distribution in solution (supernatant) and in droplets (pellets). 10 µM MeCP2 was applied for the phase separation assay in different conditions. The in vitro phase separation assay was done by incubating the mixtures at room temperature for 45 min. Then droplets were pelleted by centrifugation at 12,000 rpm for 10 min at room temperature. The top half of the supernatant was transferred to new tubes for SDS polyacrylamide gel (SDS-PAGE) electrophoresis, followed by coomassie staining and subsequent quantitative analysis. Top: Coomassie staining result of the supernatants after SDS-PAGE electrophoresis. Bottom: Quantitative analysis for SDS-PAGE gel above. Replicates (N) = 2.(F) Fluorescence images of MeCP2 phase separated droplets. The 1% GFP-MeCP2 was mixed with 99% untagged MeCP2 (molar ratio) and applied for the in vitro phase separation assay in different conditions by incubating 45 min at room temperature. Both fluorescent images (GFP) and DIC images were taken under the Nikon Eclipse TiE2 microscope equipped with differential interference contrast (DIC) microscopy. Total MeCP2 concentration: 10 µM. Scale bar = 10 µm.(G-H) Droplet aspect ratio (G) and in-droplet protein concentration (H) for experiments in F.The GFP channel was applied for droplet segmentation by bandpass filter and threshold based on the mean intensity in/out droplets. Droplets with size > 0.1 µm2 were identified and droplet parameters were measured. The aspect ratio (G) was calculated as the ratio of maximal Feret diameter to minimal Feret diameter. The in-droplet MeCP2 concentration (H) was measured by the GFP intensity inside the droplets. For details, see Fig. S2. n: Number of droplets. Replicates (N) = 3. n: number of droplets.(I) Mean values of aspect ratio and in-droplet MeCP2 concentration from (G-H).
