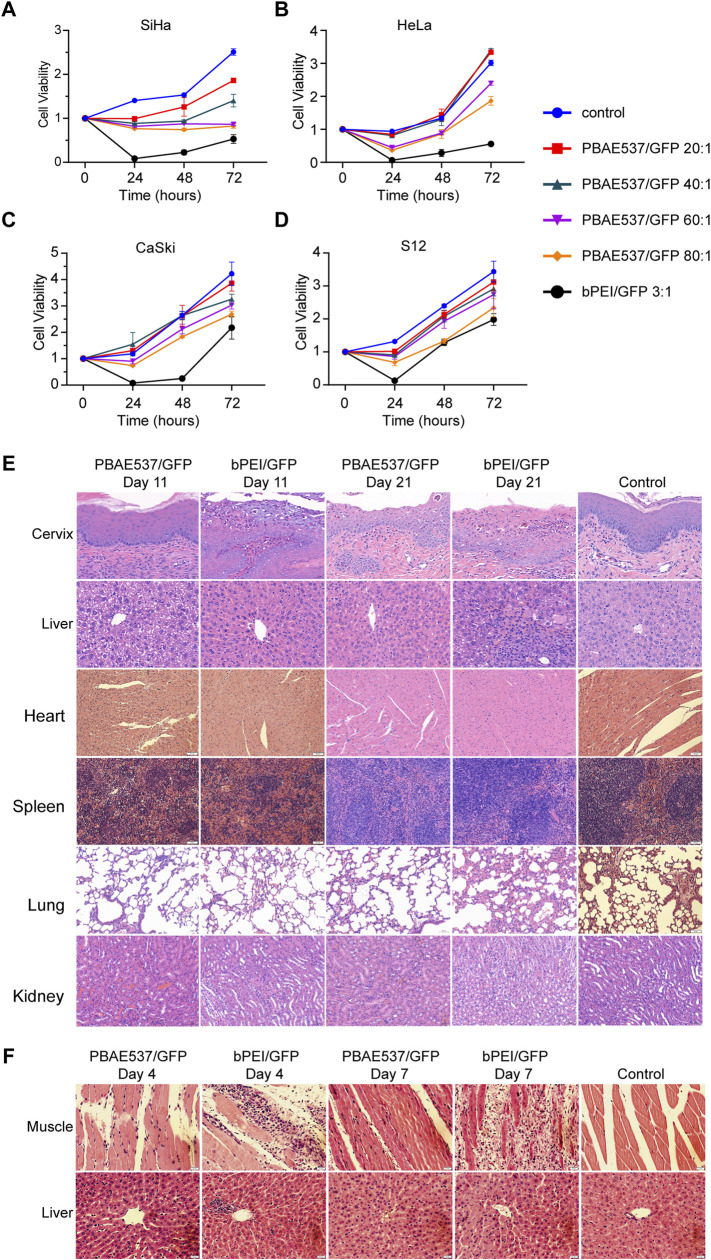
FIGURE 4.
Toxicity analysis of nanoparticles in cell lines and mouse organs. (A–D) The toxicity of PBAE537/GFP nanoparticles with different mass ratios (20:1, 40:1, 60:1, and 80:1) was detected in (A) SiHa, (B) HeLa, (C) S12, and (D) CaSki cells. bPEI/GFP (3:1) was used as a positive control. The time points of cytotoxicity were 24, 48, and 72 h after transfection. Each time point represents the mean ± SEM (n = 4). (E) HE staining of paraffin sections of cervical and liver tissues of mice that received continuous vaginal injections of PBAE537/GFP (60:1) and bPEI GFP (3:1) for 10 and 20 days. Mice were injected intravaginally with 10 μL nanoparticles containing 10 μg plasmids. Vaginal treatment was performed once daily for 10 or 20 days. Significant damage was observed only in the bPEI group, but less in the PBAE537 group. (F) Nanoparticles composed of PBAE537/GFP (60:1) and bPEI/GFP (3:1) were injected into the thigh muscles of mice for 3 consecutive days. Representative images of HE staining in paraffin sections of thigh muscle tissue and liver tissue of mice at 4 and 7 days after the initial injection. One hundred microliters of plasmids containing 100 μg nanoparticles were injected into the thigh muscles of mice every day. Scale, 20 μm.