Figure 1. The mechanism of action of GEM in PDAC treatment.
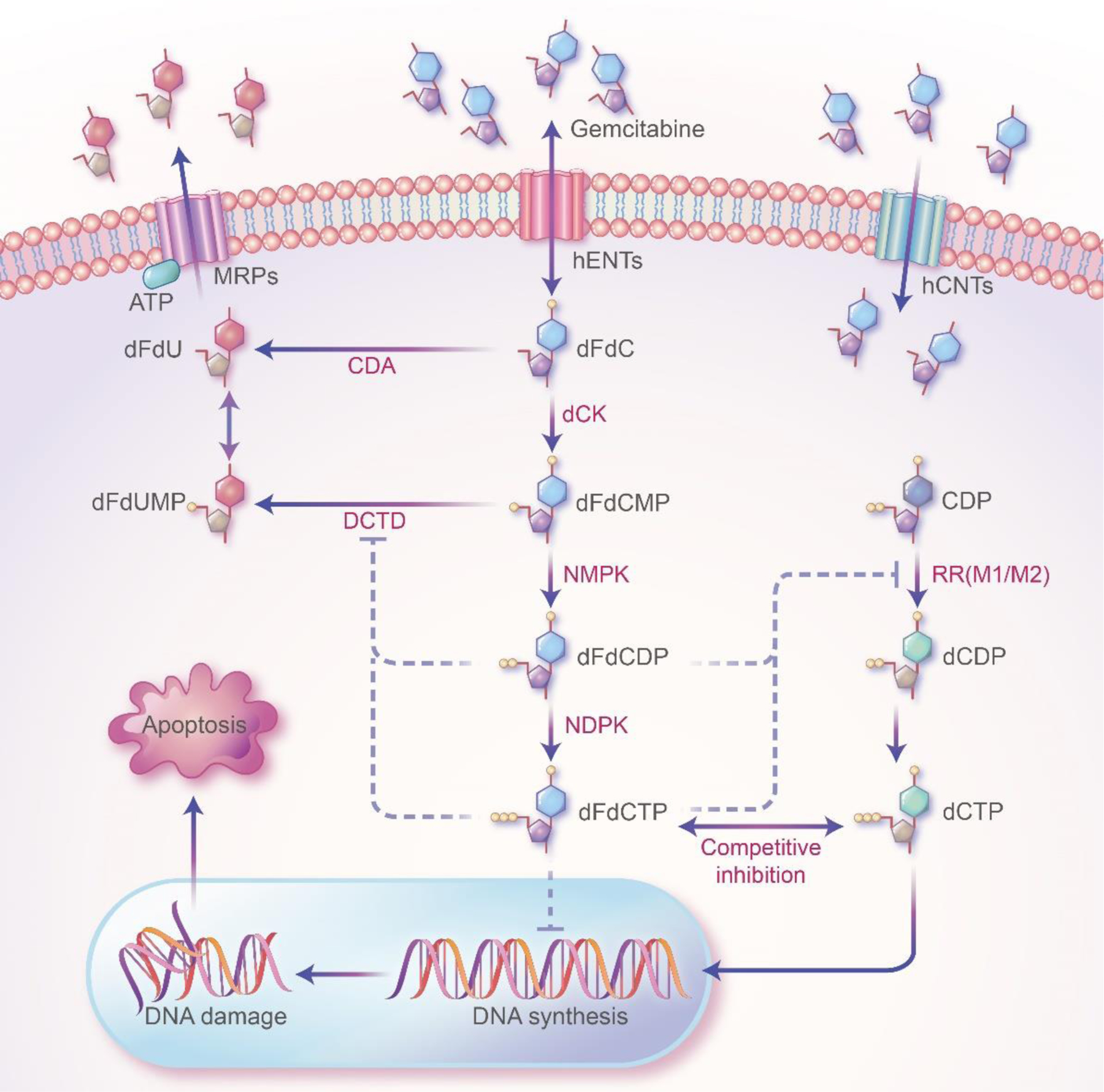
GEM (2’,2’-difluorodeoxycytidine, dFdC) crosses the cell membrane and is continuously phosphorylated by the phosphorylation rate-limiting enzyme deoxycytidine kinase (dCK) to form GEM monophosphate (dFdCMP), GEM diphosphate (dFdCDP), and GEM triphosphate (dFdCTP). As a substrate for DNA polymerase, dFdCTP competes with deoxycytidine triphosphate (dCTP), a raw material for DNA synthesis. DNA polymerase stochastically incorporates dFdCTP into DNA during replication, which terminates DNA strand elongation and ultimately leads to apoptosis. Two active metabolites, dFdCDP and dFdCTP, can inhibit ribonucleotide reductase (RR). RR is the rate-limiting enzyme (RRM1 and RRM2) in the DNA synthesis pathway and is mainly responsible for converting ribonucleotides into deoxyribonucleoside triphosphates (dNTPs), which are essential for DNA assembly and repair. In addition, dFdCDP and dFdCTP can inhibit cytidine deaminase (CDA) or deoxycytidylate deaminase (DCTD), which can inactivate dFdCMP.
