Figure 4:
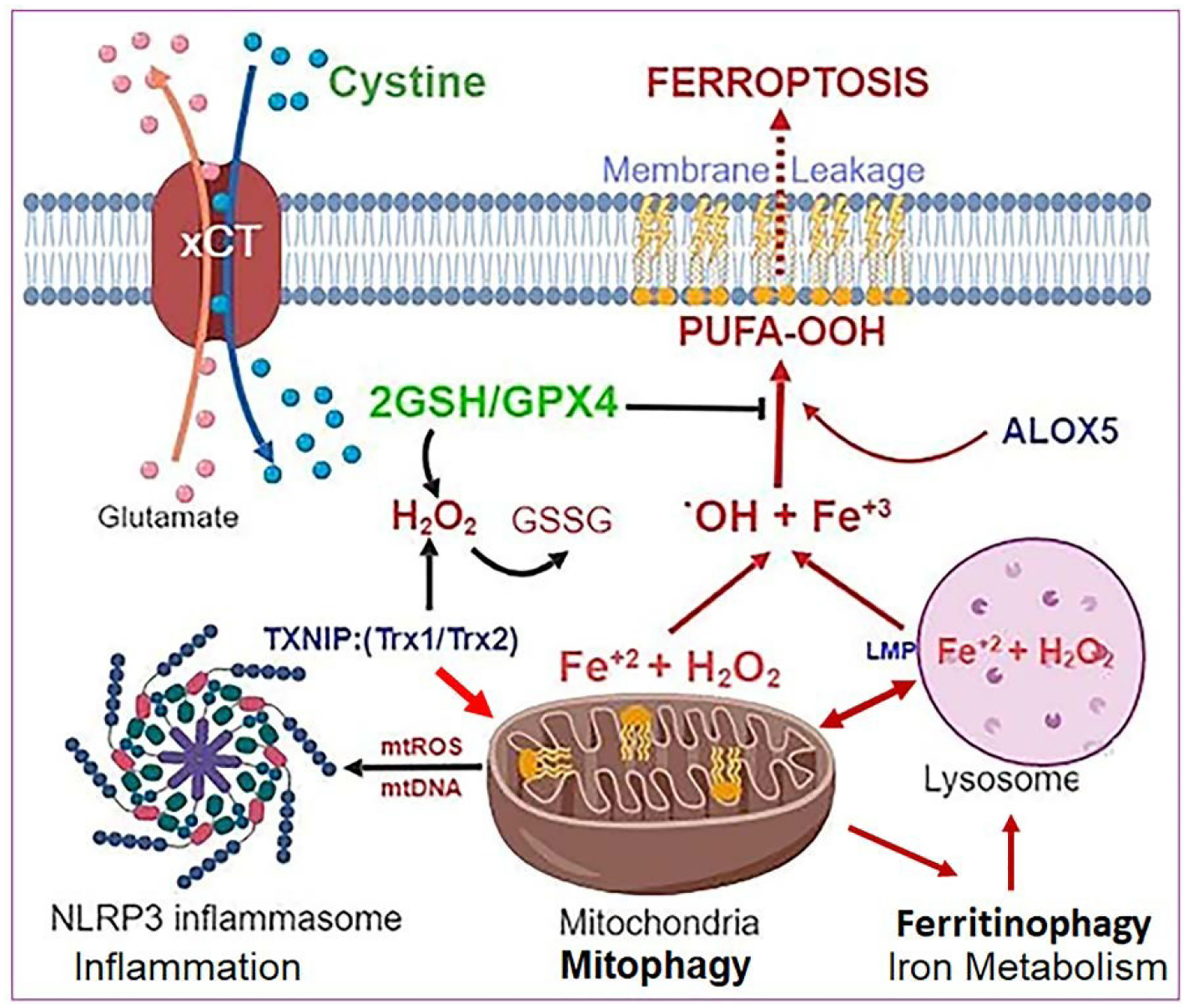
Relationship among Mitophagy, Ferritinophagy and Ferroptosis under oxidative stress. Hyperglycemia-induced TXNIP upregulation and oxidative/nitrosative stress cause mitochondrial damage, ROS generation and mitophagic flux to lysosomes for degradation. A feedback mechanism to replenish mitochondrial mass and bioenergetics may activate ferritinophagy, an autophagic process of degradation and release of free iron Fe2+ from ferritin cages in lysosomes for using in the synthesis of mitochondrial Iron-sulfur cluster and heme moeity. However, the released ferrous iron also reacts with H2O2 via Fenton reaction to generate highly reactive hydroxyl radicals (.OH), and biproducts of hydroxide (OH−) and ferric iron (Fe3+). These .OH radicals react with cell membranes rich in polyunsaturated fatty acids (PUFA) to mediate lipid peroxidation (PUFA-OOH), including the mitochondria, lysosome and plasma membranes. PUFA-OOH in membranes disturb fluidity and membrane integrity. Free Fe2+ may also activate arachidonic 5-lipoxygenase (ALOX5), which generates lipid peroxides (L- OOH). The only enzyme that can remove and prevent lipid peroxidation is GPX4, a selenocysteine protein, using 2 GSH molecules. However, under oxidative stress, GSH level and biosynthesis are down-regulated leading to accumulation of oxidized GSSG. Extracellular cystine (di-cysteine) is transported into cells through plasma membrane glutamate-cystine exchanger (xCT). Down-regulation of xCT, which occurs in DR, will limit cystine import and, therefore, cysteine available for GSH synthesis. Under these conditions, iron-dependent PUFA-OOH due to inhibition of GPX4 activity causes cell death by ferroptosis, a recently identified non-apoptotic cell death. Ferroptosis itself induces an inflammatory response, which assembles the NLRP3 inflammasome, activating caspase-1, IL- 1β/IL-18 and gasdermin-D to cause pyroptotic cell death as well. These events, if unchecked, eventually will sustain a chronic low-grade inflammation, oxidative stress and premature retinal cell death leading to disease initiation and progression of DR and age-related retinal diseases. [Image created with BioRender.com.].
