Fig. 2. Secondary metabolite interactions with oxidative stress.
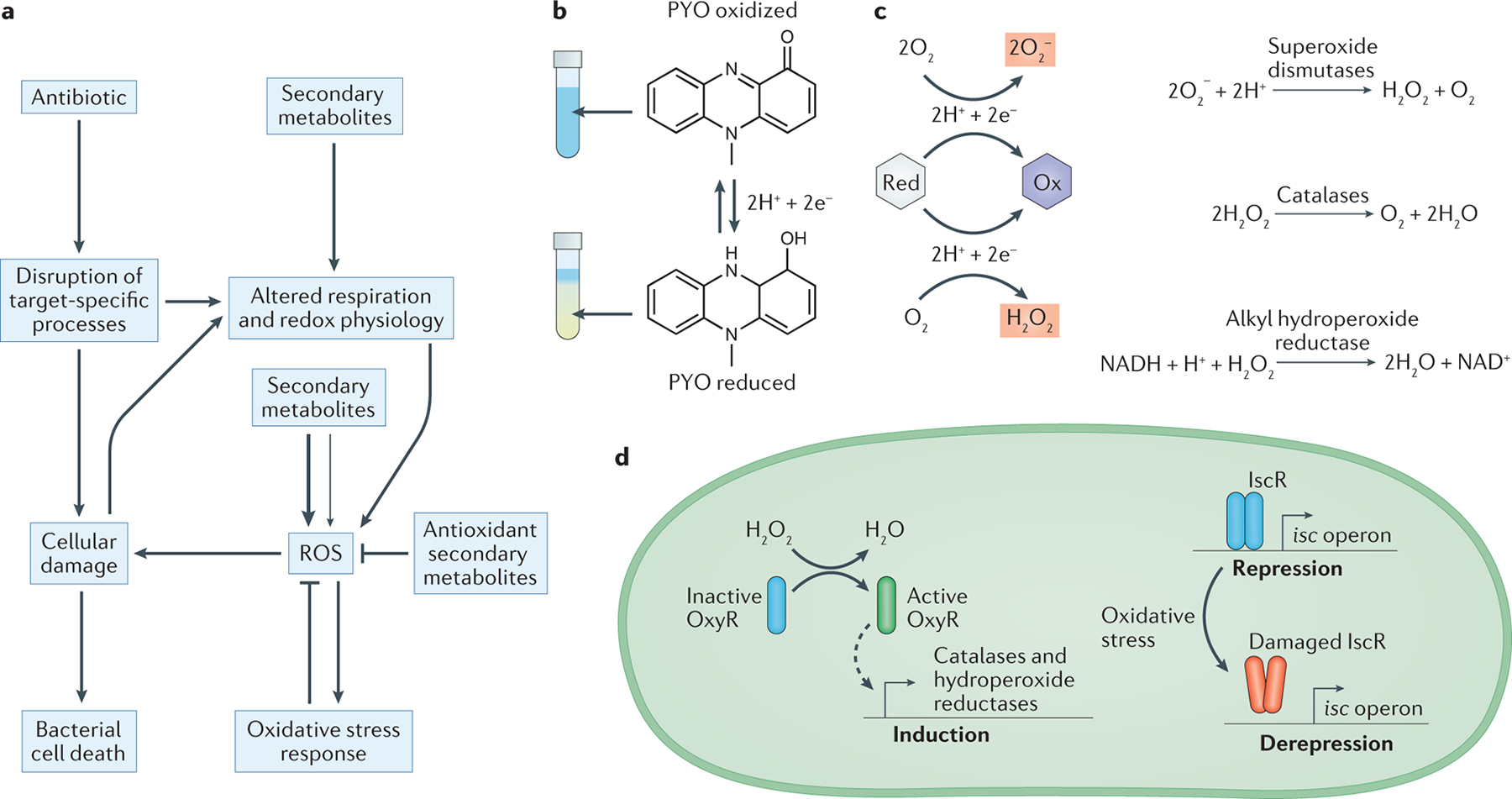
a. Schematic depicting how bactericidal antibiotics can cause cell death both by directly disrupting target-specific processes, and by indirectly promoting the formation of reactive oxygen species (ROS) as a consequence of altered respiration and cellular damage87. Secondary metabolites can interface with these pathways at multiple points, including by interfering with respiration and redox homeostasis, directly generating ROS through redox-cycling, and detoxifying ROS via one-electron reactions. Secondary metabolites that promote oxidative stress can either antagonize or potentiate antibiotic toxicity, which is likely to depend on whether the resulting increases in ROS are moderate (thin arrow) or severe (thick arrow). Moderate increases in ROS may induce protective oxidative stress responses that can counteract antibiotic toxicity, whereas severe increases in ROS may overwhelm the defenses of the cell, which leads to synergistic effects with bactericidal antibiotics. b. The redox-active nature of pyocyanin (PYO) is visually apparent as it undergoes a color change from blue (oxidized) to colorless (reduced) upon gaining two electrons and two protons from cellular reductants. This reaction is reversible under physiological conditions. c. Many redox-active secondary metabolites can donate electrons to molecular oxygen in the process of cycling from a reduced (Red) to an oxidized (Ox) state, which leads to the formation of superoxide or hydrogen peroxide. Cells can detoxify these forms of ROS through enzymatic reactions catalyzed by superoxide dismutase, catalase, and alkyl hydroperoxide reductase162. d. Bacterial oxidative stress responses are typically regulated through multiple pathways. For example, in Pseudomonas aeruginosa, the H2O2-sensing transcription factor OxyR controls the expression of catalases and alkyl hydroperoxide reductases (AHPs)168,169 (left). In addition, the transcription factor iron-sulfur cluster regulator (IscR) upregulates the biosynthesis of iron–sulfur cluster (isc) operons in response to oxidative stress caused by ROS such as superoxide or H2O2, which can directly damage the iron–sulfur cluster in IscR itself and thereby lead to depression of its regulon170.
