Figure 7. The adjuvant formulation of mannans and alum confers protection against lung viral infections.
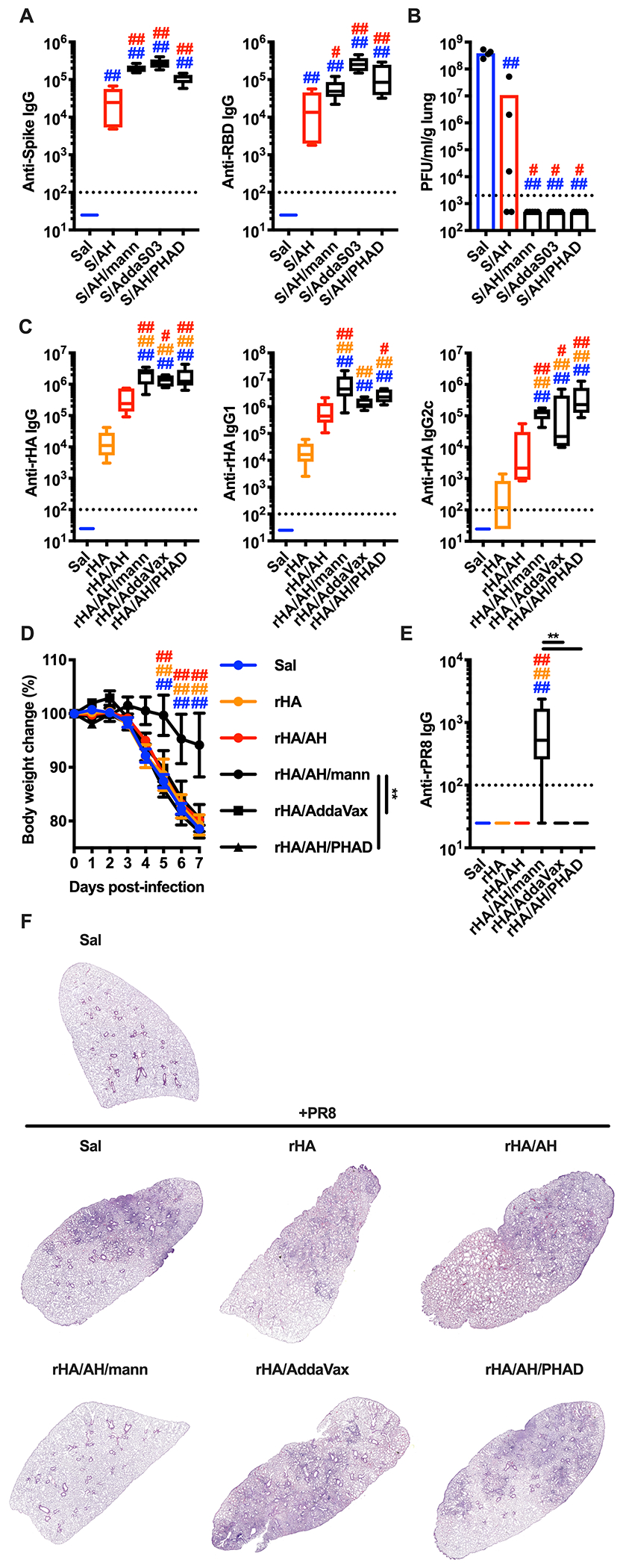
(A, B) Mice were injected intradermally with saline (Sal), pre-fusion stabilized SARS-CoV-2 trimer alone (S) or combined with alum (AH) (S/AH), AH/mannans (S/AH/mann), AddaS03 (S/AddaS03), or AH/PHAD (S/AH/PHAD) on day 0 (prime) and day 14 (boost). Serum samples were collected on day 28 to assess anti-Spike and anti-RBD antibody levels (A). On day 35 mice were intranasally infected with SARS-CoV-2 MA10 on day 35 and 2 days later numbers of plaque forming units (PFU) were quantified in the lungs (B). N = 4-5 mice per group. (C - F) Mice were injected intradermally with saline (Sal), Flublok alone (rHA) or combined with AH (rHA/AH), AH/mannans (rHA/AH/mann), AddaVax (rHA/AddaVax), or AH/PHAD (rHA/AH/PHAD) on day 0 (prime) and day 14 (boost). Serum samples were collected on day 28 to assess antibodies against rHA (anti-rHA, C) or IAV A/PR/8/1934 recombinant hemagglutinin (anti-rPR8, E). On day 35 mice were intranasally infected with IAV A/PR/8/1934 and body weights were recorded for 7 days (D). N = 5 (C, E) or 8 (D) mice per group. On day 7 post-infection mice were sacrificed and lungs were collected for histological analysis (hematoxylin eosin staining, F). One representative image per group is shown. #, * and ##, ** respectively indicate p ≤ 0.05 and 0.01. Comparisons are indicated by the color code. See also Figure S7.
