Abstract
Purpose
Orbital teratoma can be removed in order to preserve the bulb.
Observations
Case report of a newborn with an orbital tumor. After spontaneous birth, a massive bulbus protrusion on the left side was observed. Magnetic Resonance Imaging (MRI) diagnosis showed an intraorbital cystic lesion containing solid parts and displacing the bulbus oculi. Suspecting a teratoma, primarily a cystic puncture was performed on the first day of life. On the 3rd day of life, cystic lesion was completely resected while preserving the bulbus. Histologically a mature cystic teratoma was observed.
Conclusion and Importance
This case shows how important prenatal diagnostics is in order to plan the necessary birth preparations in advance and that a bulbus-preserving surgery in orbital teratoma is possible. In the absence of yolk-salk tumor it is associated with a good prognosis.
Keywords: Orbital tumor, Teratoma, Pathology, Congenital tumor
1. Introduction
Teratomas are the most common intracranial tumors in newborns.1 However, localization in the orbit is very rare with 0.8%.2 They consist of all three germinal layers and are mostly well differentiated and benign.3 Malignant teratomas have rarely been described.4 In the literature, exenterating of the bulb is reported as primary therapy in individual case reports, but is rather realized only after complications such as exposure keratopathy and perforation.4, 5, 6 In our case as also described in literature,7 the teratoma could be removed in order to preserve the bulb.
2. Case report
2.1. Clinical history
A female baby was born at term after an uneventful pregnancy, including a prenatal ultrasound examination of the head in the 30th week which showed no anomaly. The newborn was the fourth child of non-consanguineous parents of Tunisian origin; the family history revealed no hereditary diseases. At birth, a massive protrusio bulbi on the left side was present.
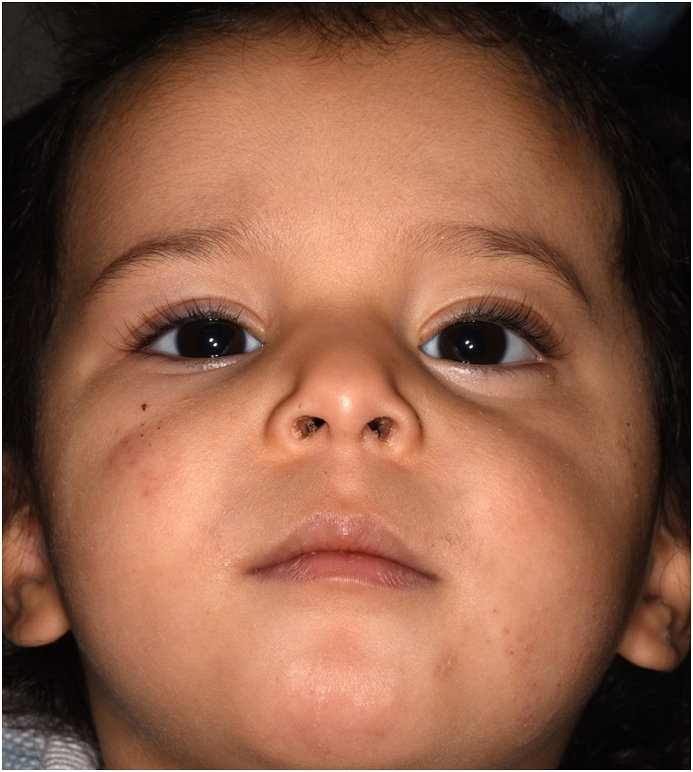
The ophthalmological examination showed a large protrusio and displacement of the left bulbus towards the superior nasal side (Fig. 1A). A more in depth examination was not possible even with manual eyelid opening, due to the massive displacement. The anterior part of the right eye was normal. Due to neurological monitoring of the pupil light reaction, fundoscopy could not be performed on the first day of life. The general neonatological examination revealed no other pathologic findings or tumorous disease.
Fig. 1.
A At birth the eye globe (x) is luxated cranially and medially out of the Orbit.
B After surgery the eye globe returned into correct vertical position.
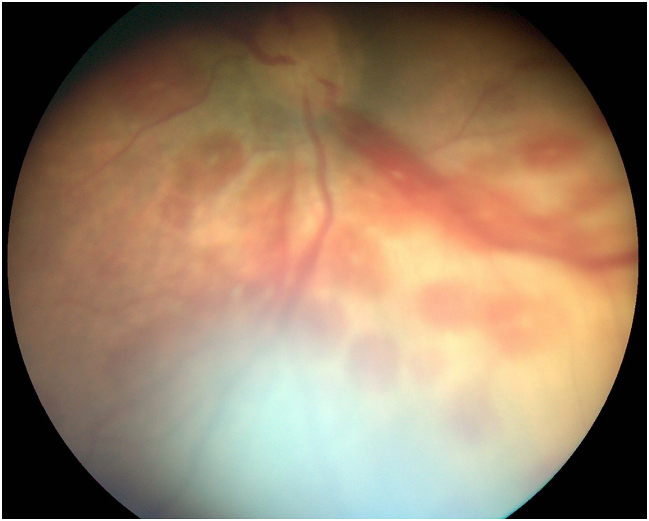
C Fundus picture on 2nd day of life with bleeding, right eye.
D Fundus picture on 2nd day of life with bleeding, left eye.
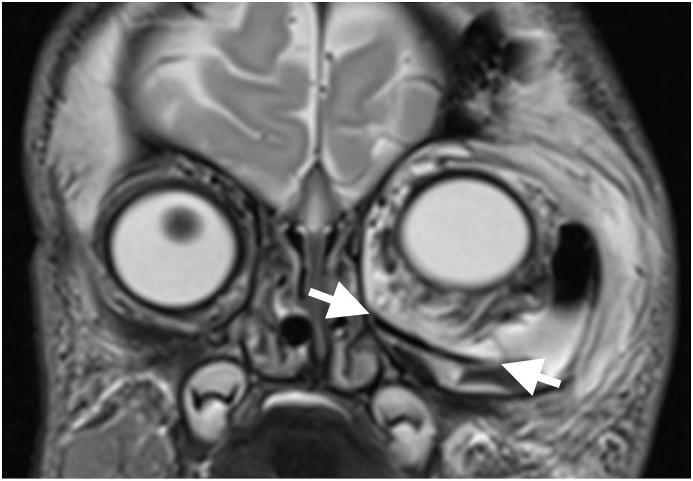
The MRI of the skull, performed under general anesthesia on the first day of life, showed a partly cystic, partly solid intra-orbital lesion displacing the optic nerve and the vessels to the nasal side (Fig. 2A). To relieve this extreme displacement, orbital decompression was performed by a trans-conjunctival access immediately thereafter. Eight milliliters of an amber colored liquid were punctured. After the procedure, the bulbus was moved to an origin position and the left pupil showed reaction to light exposure.
Fig. 2.
A The cystic lesion contains liquid (l) and solid (s) components and is in contact to the optic nerve (*).
B A cranial vault fragment has been used to form the orbital floor (→ ←).
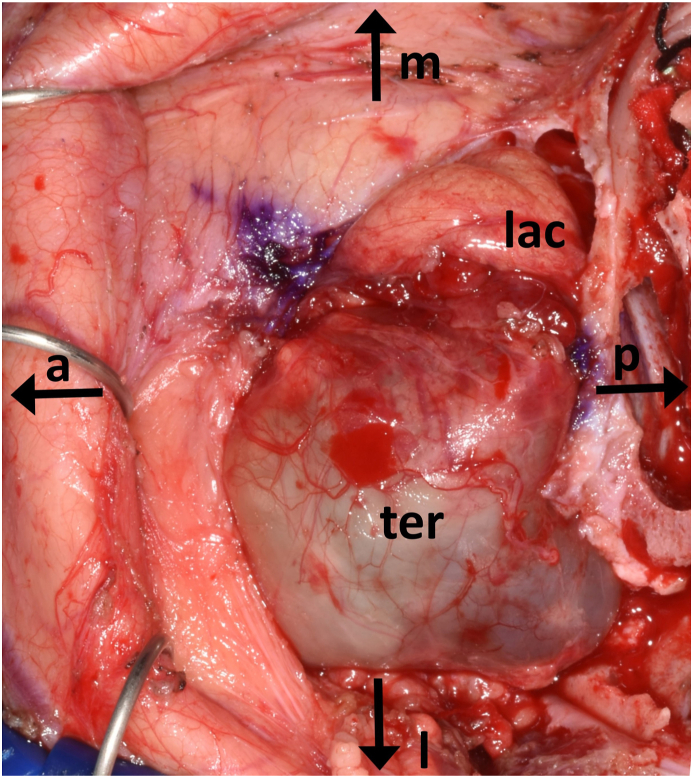
C Intraoperative findings after coronal access with frontal craniotomy. The teratoma occupies the space of the lateral orbit with resorption of the lateral orbital wall and orbital floor (a, anterior; p, posterior; m, medial; l, lateral; lac, lacrimal gland; ter, teratoma).
Cytological, a cell population positive for the pancytokeratin marker CK22 was found, suggesting epithelial cells of unknown origin. This finding was compatible with the presence of a teratoma and made a glial lesion unlikely. The evidence of positive beta trace protein indicated parts of cerebrospinal fluid.
On the 2nd day of life, fundoscopy showed a bilateral central praeocclusion with intra-retinal flame hemorrhage (Fig. 1C and D). MRI angiography excluded a cavernosus sinus venous thrombosis.
After an interdisciplinary discussion with colleagues from maxillofacial surgery, pediatric neurosurgery, neonatology and pediatric oncology, a tumor resection with bulbus preservation was planned on for the third day of life. A small frontal-orbito-zygomatic approach was used to access the orbit with the aid of neuronavigation (Brianlab, Munich, Germany). After ultrasound imaging of the cyst and bulbus, the cystic sac was bluntly freed while protecting the eye muscles. To gain further access to the caudal circumference of the cyst, the sac was opened to release its tension. This allowed for complete macroscopically mobilization and enucleation of the cyst (Fig. 2, Fig. 3D). The intraoperative frozen section examination confirmed the suspicion of a mature teratoma. Due to the expansive growth of the cystic lesion, the orbital floor and lateral orbital wall were missing at the time of surgery. The eroded and enlarged orbital space and bone were reconstructed using autologous bone replacement gained from a small frontal craniotomy (10 × 20 mm) in order to avoid enophtalmos in the future (Fig. 2B). Subsequently, the frontal craniotomy bone fragment was fixed with resorbable plates and pins, the temporal muscle was refixated and the skin was closed in ordinary fashion using resorbable sutures. In the postoperative MRI, no residual tumor was seen. The infant showed a full clinical recovery, while the peri-postoperative blood loss was minimal, and no blood transfusions were needed.
Fig. 3.
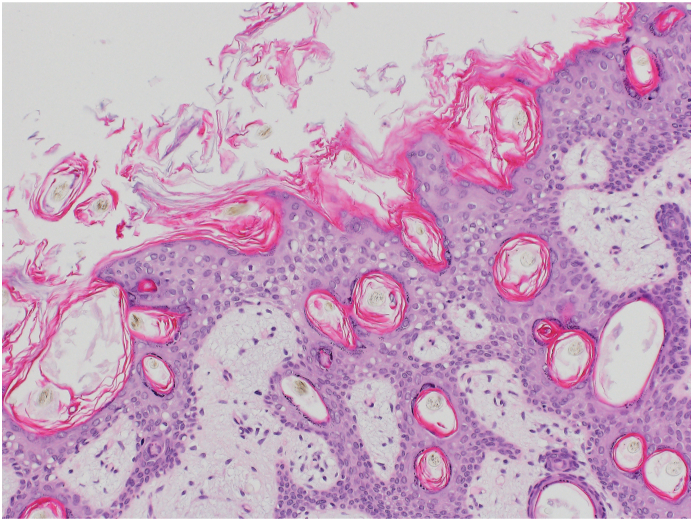
A Histology: Tissue of all three germinal layers: Mature bone tissue.
B Histology: Mature keratinizing squamous epithelium with mature adnexal structure.
C Histology: Cyst lined with not-keratinizing multilayered epithelium.
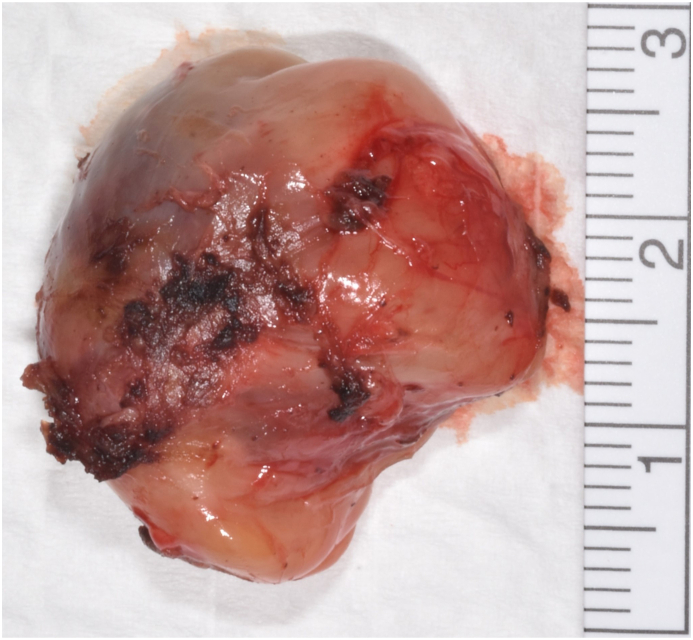
D Enucleated cyst, view from outside.
Lid opening was possible spontaneously, allowing occlusion therapy to be started in order to avoid amblyopia. The patient was discharged with analgesics and a topical steroid therapy, which could be stopped after 2 weeks.
Alpha-fetoprotein (AFP) and beta-HCG are useful biomarkers in malignant germ cell tumors. In our case, AFP at diagnosis was high but in the normal range for a newborn (11′394 klU/L, ref. < 5.8 klU/L). In the follow-up, AFP levels constantly decreased, as expected, with a follow-up at 4 months of age showing a value of 70.3 kIU/L. Beta-HCG was always in the normal range (<0.8 lU/L, ref. < 3 IU/L).
2.2. Follow up
Three months postoperatively, a control MRI showed a fully normal brain parenchyma with age-appropriate myelination; there was no evidence of residual tumor or local relapse. The retinal hemorrhage was completely resorbed. Six months postoperatively, a slight regression of the left abductor nerve palsy was observed. There was residual asymmetry of the lid fissure and minimal anisocoria (about 1 mm) with relative mydriasis of the left pupil with preserved reflex to light exposure. The baby fixed the left lateral field and could move her left eye over the midline. The visual acuity equivalent was symmetrical. The occlusion trial showed symmetrical a good acceptance. Amblyopia could not be definitively ruled out, but a large refractive asymmetry was considered unlikely to develop. The occlusion therapy was temporarily stopped for symmetrical visual acuity. The fronto-temporal skin incision was completely healed, the resected frontal bone piece and the craniotomy showed very good ossification, leading to an overall good cosmetic result (Fig. 1B).
2.3. Ophthalmic pathology
Macroscopically, a predominantly cystic lesion of 15 × 29 × 15 mm was observed (Fig. 3D). Histologically the excised tissue contained parts of compact collagenous tissue, parts of fatty tissue as well as parts of a cyst sac lined with a multilayered, non-keratinizing squamous cell epithelium (Fig. 3B and C). Furthermore, smaller cysts lined with plexus choroideus or gastrointestinal epithelium with multiple goblet cells and secretion with strong positive staining of periodic Acid-Schiff (PAS) were present. Lymphocytic infiltration was found in the surrounding stromal tissue. In addition, bone tissue (Fig. 3A), musculature and cartilage were found. Immature neural or yolk-sac tumor suspicious structures were not found. There was no clear-cut positivity for AFP, Glypikan 3 was negative.
2.4. Final histological diagnosis
Mature cystic teratoma, G0 according to Gonzales-Crussi grading system was observed. No immature or yolk-sac tumor suspicious structures seen. No clear-cut AFP-staining.
3. Discussion
This case report describes a newborn with a huge mature intraorbital teratoma, which could be removed in toto with preservation of the eye bulb. The first line therapy of mature teratomas is early surgical resection due to their potentially rapid local growth and benign histology. Interestingly, in our case but also in another case report,6 the huge protrusio bulbi was not suspected prenatally. In both cases, the last antenatal ultrasound was performed routinely around the 30th week of pregnancy and showed no anomaly; this points out the fact that these lesions can grow massively during the third trimester.
Due to the expansive growth of the cystic lesion the orbital floor and lateral orbital wall were missing at the time of surgery. To restore binocular vision and fronto-orbital skull growth, the symmetric reconstruction of the bony orbital vault is important. Free full-thickness autologous bone grafts from the parietal calvaria have been used to reconstruct the orbital floor and lateral orbital rim. The grafts were fixed simply with slow-resorbing sutures (polydioxane). No titanium fixation plates were used since growth related bone remodeling leads to uncontrollable and potential intracranial migration of non-absorbable foreign material. Calvarial bone donor sites undergo full spontaneous bone regeneration if performed in the first year of life due to the bone inductive nature of the underlying dura – thus an exclusive ability of the neurocranium but absent in the midfacial skeleton.
Complete surgical cyst removal is curative but may have significant complications. First, nerve palsies may ensue, but are usually transient. Nevertheless, they must be recognized, so that occlusion therapy of the healthy eye can be started early to avoid future amblyopia. Second, optic nerve compression (through the mass effect) or lesion (through manipulation during surgery) may lead to vision impairment. It is therefore critical to implement tight ophthalmological follow-up to detect asymmetry in the visual acuity. Further complications of bulbus-saving surgery are described in the literature such as diplopia, bony defects and facial growth disorders, among others.6 Close interdisciplinary collaboration between ophthalmologists, pediatric neurosurgeons and maxillofacial surgeons, with specific pediatric surgical expertise, is mandatory in order to achieve the best results in terms of long-term oncological control as well as orbital development with visual rehabilitation.
On the second day of life, a central vein preaocclusion with pronounced flame hemorrhage appeared surprisingly on both sides, showing complete spontaneous regression. Initially a plausible cause of a sinus vein thrombosis was excluded with MRI. Possibly the bleeding occurred due to the orbital mass in combinations with pressure under spontaneous delivery. We recommend to go for cesarean section if these pathologies are detected intra-uterine.
Teratomas are defined as germ cell tumors, composed of elements of ectodermal, mesodermal and endodermal element. Grading of gonadal and extragonadal teratomas follow the degree of maturity of tissues as compared to the age of individual patient. Most congenital teratomas, also in the orbit are mature.5,8 The cystic architecture is predominant. For a diagnosis of mature teratoma all tissue components must be mature. Immature teratomas contain immature neuroepithelial tissue.9 Sarcomatous elements are considered as a poor prognostic factor in gonadal teratomas, as are yolk sack tumor component.10 Prenatal general biology is evolving as a crucial background.10
Postoperatively there was a sixth nerve palsy on the left side and therefore an amblyopia therapy was initiated. At six months follow up a slight regression of the left nerve palsy and a symmetrical visual acuity was shown. The significance of the diagnostic detection of AFP in serum is controversially discussed.11 According to a review by Morreddu et al., the preoperative AFP concentration is a good prognostic marker for newborn with teratomas.6
4. Conclusion
This case shows how important prenatal diagnostics is in order to plan the necessary birth preparations in advance, as was unfortunately not possible in our case. The case shows that a bulbus-preserving surgery is possible and in the absence of yolk-salk tumor is associated with a good prognosis. The initial motility disorder is decreasing after one year and currently does not require occlusion therapy because of symmetrical visual acuity. However, close controls are essential.
Funding
No funding or grant support.
Submission declaration
The work described has not been published previously.
Patient consent to publication
Informed written consent was obtained from the patient's legal guardian for publication of personal and medical record details.
Authorship contributions
Aja Reinhold: Conceptualization; Data curation; Formal analysis; Investigation; Project administration; Resources; Visualization; Writing, Review and Editing. Peter Meyer: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Validation, Writing, Review and Editing. Elisabeth Bruder: Investigation; Resources; Writing, Review and Editing. Jehuda Solemann: Investigation; Review and Editing. Andreas Müller: Investigation; Review and Editing. Nicolas von der Weid: Data curation; Formal analysis; Investigation; Project administration; Resources; Writing, Review and Editing. Miodrag Savic: Investigation; Review and Editing.
Declaration of competing interest
(AR, PM, EB, JS, NW, AM, MS) none.
The authors attest that they have no financial or other conflict of interest.
References
- 1.Isaacs H., Jr. Perinatal brain tumors: a review of 250 cases. Pediatr Neurol. 2002;27(4):249–261. doi: 10.1016/s0887-8994(02)00472-1. [DOI] [PubMed] [Google Scholar]
- 2.Gunalp I., Gunduz K. Cystic lesions of the orbit. Int Ophthalmol. 1996;20(5):273–277. doi: 10.1007/BF00131923. [DOI] [PubMed] [Google Scholar]
- 3.Tapper D., Lack E.E. Teratomas in infancy and childhood. A 54-year experience at the children's hospital medical center. Ann Surg. 1983;198(3):398–410. doi: 10.1097/00000658-198309000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prakash M.V., Indira R., Radhakrishnan M., Leela G. Malignant orbital teratoma in a neonate: a clinicopathological case report. J Postgrad Med. 2017;63(3):203–205. doi: 10.4103/jpgm.JPGM_629_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pellerano F., Guillermo E., Garrido G., Berges P. Congenital orbital teratoma. Ocul Oncol Pathol. 2017;3(1):11–16. doi: 10.1159/000448144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreddu E., Pereira J., Vaz R., Lena G., Triglia J.M., Nicollas R. Combined endonasal and neurosurgical resection of a congenital teratoma with pharyngeal, intracranial and orbital extension: case report, surgical technique and review of the literature. Int J Pediatr Otorhinolaryngol. 2015;79(12):1991–1994. doi: 10.1016/j.ijporl.2015.10.056. [DOI] [PubMed] [Google Scholar]
- 7.Oommen J., Mohammed H., Ayyappan Kutty S., et al. Neonatal teratoma: craniofacial treatment. J Craniofac Surg. 2019;30(1):e17–e19. doi: 10.1097/SCS.0000000000004906. [DOI] [PubMed] [Google Scholar]
- 8.Lack E.E. Extragonadal germ cell tumors of the head and neck region: review of 16 cases. Hum Pathol. 1985;16(1):56–64. doi: 10.1016/s0046-8177(85)80214-8. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Crussi F. second ed. Armed Forces Institute of Pathology; Washington DC: 1982. Extragonadal Teratomas. [Google Scholar]
- 10.Calaminus G., Schneider D.T., von Schweinitz D., et al. Age-dependent presentation and clinical course of 1465 patients aged 0 to less than 18 Years with ovarian or testicular germ cell tumors; Data of the MAKEI 96 protocol revisited in the light of prenatal germ cell biology. Cancers. 2020;12(3) doi: 10.3390/cancers12030611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuchida Y., Hasegawa H. The diagnostic value of alpha-fetoprotein in infants and children with teratomas: a questionnaire survey in Japan. J Pediatr Surg. 1983;18(2):152–155. doi: 10.1016/s0022-3468(83)80538-7. [DOI] [PubMed] [Google Scholar]