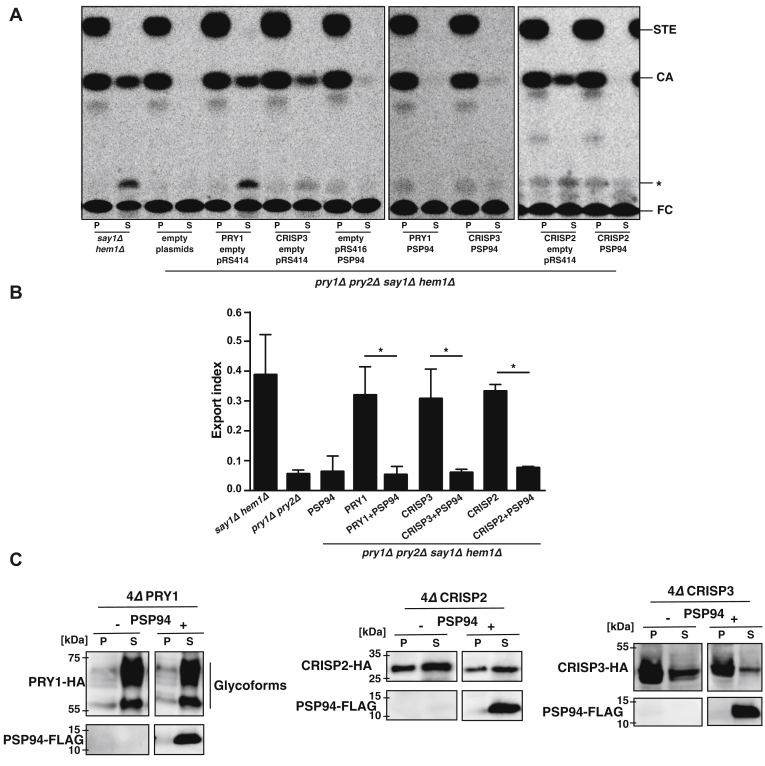
Figure 1.
PSP94 inhibits sterol export by CAP proteins.A, export of acetylated cholesterol is blocked in cells expressing PSP94. Acetylation and export of [14C]cholesterol were examined in hem1Δ say1Δ double mutant cells and in quadruple mutant cells lacking the endogenous CAP family members Pry1 and Pry2 (pry1Δ pry2Δ hem1Δ say1Δ). Strains expressing the indicated CAP protein from a plasmid (PRY1, CRISP2, or CRISP3) and coexpressing either PSP94 or an empty control plasmid, pRS414, were cultivated in the presence of [14C]cholesterol. Lipids were extracted from cell pellet (P) and culture supernatant (S), separated by TLC, and visualized by phosphorimaging. The position of free cholesterol (FC), cholesteryl acetate (CA), steryl esters (STE), and an unidentified lipid (∗) are indicated to the right. B, quantification of cholesteryl acetate export. The plotted export index indicates the relative levels of cholesteryl acetate exported by the cells. The export index corresponds to the ratio of extracellular cholesteryl acetate to the sum of intracellular and extracellular cholesteryl acetate. Data correspond to means ± SD of three independent experiments, and statistical significance is indicated: ∗p ≤ 0.05. C, expression of PSP94 does not block the synthesis or secretion of CAP family members. Proteins were precipitated using trichloroacetic acid from the culture medium (S) and cells (P) expressing HA-tagged Pry1, CRISP2, or CRISP3 in the presence or absence of FLAG-tagged PSP94 and analyzed by Western blotting.