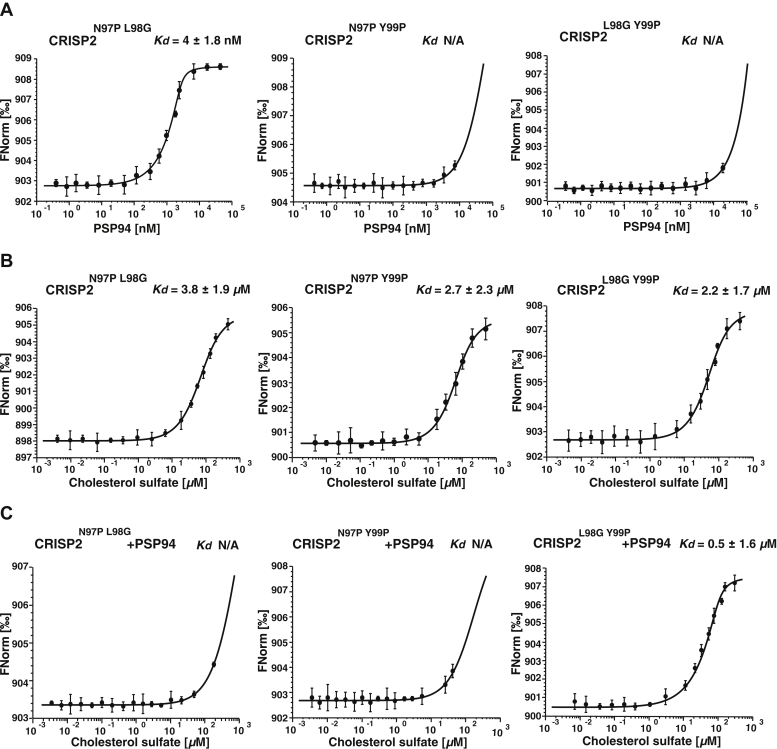
Figure 5.
Identification of two key residues within CRISP2 that block the interaction with PSP94 but not binding of cholesterol.A–C, all three double mutant combinations of CRISP2 in the three residues that are predicted to interact with PSP94, N97, L98, and Y99 were generated. The mutant proteins were expressed in E. coli and purified. Their binding to PSP94 (A), cholesterol sulfate (B), and cholesterol sulfate in the presence of PSP94 (C) was assessed by microscale thermophoresis. The CRISP2L98G Y99P double mutant is the sole of the three double mutant versions that retained the capacity to bind cholesterol sulfate even in the presence of PSP94. Measurements were performed in triplicates, and the corresponding dissociation constants (Kd) are indicated. N/A, not applicable.