Key Points
Question
Does targeting programmed cell death 1 or programmed cell death ligand 1 enhance the clinical benefits of anti–vascular endothelial growth factor therapy in patients with refractory metastatic colorectal cancer (mCRC)?
Findings
In this randomized clinical trial of 128 patients with refractory mCRC, the addition of atezolizumab to capecitabine and bevacizumab therapy led to marginally longer progression-free survival (PFS) compared with placebo (median, 4.4 months vs 3.6 months). Although this result met the prespecified threshold for statistical significance, the improvement in PFS was not clinically relevant.
Meaning
In this study, the addition of atezolizumab to capecitabine and bevacizumab therapy provided limited clinical benefit for patients with refractory mCRC, suggesting that cotargeting programmed cell death 1 or programmed cell death ligand 1 and vascular endothelial growth factor may not meaningfully improve PFS among a population with refractory mCRC.
Abstract
Importance
Cotargeting vascular endothelial growth factor and programmed cell death 1 or programmed cell death ligand 1 may produce anticancer activity in refractory metastatic colorectal cancer (mCRC). The clinical benefit of atezolizumab combined with chemotherapy and bevacizumab remains unclear for the treatment of mCRC.
Objectives
To assess whether the addition of atezolizumab to capecitabine and bevacizumab therapy improves progression-free survival (PFS) among patients with refractory mCRC and to perform exploratory analyses among patients with microsatellite-stable (MSS) disease and liver metastasis.
Design, Setting, and Participants
This double-blind phase 2 randomized clinical trial enrolled 133 patients between September 25, 2017, and June 28, 2018 (median duration of follow-up for PFS, 20.9 months), with data cutoff on May 4, 2020. The study was conducted at multiple centers through the Academic and Community Cancer Research United network. Adult patients with mCRC who experienced disease progression while receiving fluoropyrimidine, oxaliplatin, irinotecan, bevacizumab, and anti–epidermal growth factor receptor antibody therapy (if the patient had a RAS wild-type tumor) were included.
Interventions
Patients were randomized (2:1) to receive capecitabine (850 or 1000 mg/m2) twice daily on days 1 to 14 and bevacizumab (7.5 mg/kg) on day 1 plus either atezolizumab (1200 mg; investigational group) or placebo (placebo group) on day 1 of each 21-day cycle.
Main Outcomes and Measures
The primary end point was PFS; 110 events were required to detect a hazard ratio (HR) of 0.65 with 80% power (1-sided α = .10). Secondary end points were objective response rate, overall survival (OS), and toxic effects.
Results
Of 133 randomized patients, 128 individuals (median age, 58.0 years [IQR, 51.0-65.0 years]; 77 men [60.2%]) were assessed for efficacy (82 in the investigational group and 46 in the placebo group). Overall, 15 patients (11.7%) self-identified as African American or Black, 8 (6.3%) as Asian, 1 (0.8%) as Pacific Islander, 101 (78.9%) as White, 1 (0.8%) as multiple races (Asian, Native Hawaiian/Pacific Islander, and White), and 2 (1.6%) as unknown race or unsure of race. Microsatellite-stable disease was present in 110 patients (69 in the investigational group and 41 in the placebo group). Median PFS was 4.4 months (95% CI, 4.1-6.4 months) in the investigational group and 3.6 months (95% CI, 2.2-6.2 months) in the placebo group (1-sided log-rank P = .07, a statistically significant result; HR, 0.75; 95% CI, 0.52-1.09). Among patients with MSS and proficient mismatch repair, the HR for PFS was 0.66 (95% CI, 0.44-0.99). The most common grade 3 or higher treatment-related adverse events in the investigational vs placebo groups were hypertension (6 patients [7.0%] vs 2 patients [4.3%]), diarrhea (6 patients [7.0%] vs 2 patients [4.3%]), and hand-foot syndrome (6 patients [7.0%] vs 2 patients [4.3%]). One treatment-related death occurred in the investigational group. In the investigational group, the response rate was higher among patients without liver metastasis (3 of 13 individuals [23.1%]) vs with liver metastasis (4 of 69 individuals [5.8%]). The benefit of atezolizumab for PFS and OS was greater among patients without vs with liver metastasis (primary analysis of PFS: HR, 0.63 [95% CI, 0.27-1.47] vs 0.77 [95% CI, 0.51-1.17]; OS: HR, 0.33 [95% CI, 0.11-1.02] vs 1.14 [95% CI, 0.72-1.81]).
Conclusions and Relevance
In this randomized clinical trial, the addition of atezolizumab to capecitabine and bevacizumab therapy provided limited (ie, not clinically meaningful) clinical benefit. Patients with MSS and proficient mismatch repair tumors and those without liver metastasis benefited more from dual inhibition of the vascular endothelial growth factor and programmed cell death 1 or programmed cell death ligand 1 pathways.
Trial Registration
ClinicalTrials.gov Identifier: NCT02873195
This randomized clinical trial assesses whether the addition of atezolizumab to capecitabine and bevacizumab therapy improves progression-free survival among patients with refractory metastatic colorectal cancer.
Introduction
Combinatorial strategies to enhance the efficacy of anti–programmed cell death 1 (PD-1) and anti–programmed cell death ligand 1 (PD-L1) antibodies in metastatic colorectal cancer (mCRC) are needed. Targeting PD-1 and PD-L1 (PD-1/PD-L1) improves clinical outcomes among patients with microsatellite instability–high (MSI-H) and deficient mismatch repair (dMMR) mCRC1,2,3,4; however, no clinical benefit has been observed among those with microsatellite-stable (MSS) and proficient mismatch repair (pMMR) mCRC.1,5 The identification of therapeutic combinations that enhance the clinical activity of anti–PD-1/PD-L1 antibodies in mCRC represents a huge unmet need.
The vascular endothelial growth factor (VEGF) family plays a critical role in angiogenesis and may contribute to immune checkpoint inhibitor resistance.6,7,8 Antiangiogenic agents, such as bevacizumab, normalize tumor vasculature; this normalization increases T-cell infiltration and hampers immunosuppressive cells.8,9 Dual inhibition of the VEGF and PD-1/PD-L1 axes has resulted in therapeutic activity in multiple tumor types.10,11,12,13 Among patients with MSI-H and dMMR (MSI-H/dMMR) mCRC, an objective response rate (ORR) of 30% (all partial responses) and a disease control rate of 90% were observed with bevacizumab plus atezolizumab therapy in a safety extension of the phase 1b GP28328 study (Study of the Safety and Pharmacology of Atezolizumab [Anti–PD-L1 Antibody] Administered With Bevacizumab and/or Chemotherapy in Patients With Advanced Solid Tumors).14,15 Despite the dual VEGF and PD-1/PD-L1 inhibition activity observed in the refractory setting, treatment with induction FOLFOX (leucovorin calcium, fluorouracil, and oxaliplatin) and bevacizumab followed by maintenance fluoropyrimidine and bevacizumab with or without atezolizumab showed no differences in progression-free survival (PFS) or overall survival (OS) in the maintenance setting among patients with mCRC.16 Thus, the clinical benefit of atezolizumab combined with chemotherapy and bevacizumab remains unclear for the treatment of mCRC.
In this double-blind placebo-controlled multicenter phase 2 randomized clinical trial, we evaluated the combination of capecitabine and bevacizumab (capecitabine/bevacizumab) with or without atezolizumab for the treatment of patients with refractory mCRC. The primary end point was PFS. Secondary end points were ORR, OS, and toxic effects. The final results from the phase 2 randomized clinical trial are reported in this article.
Methods
Study Design and Population
The BACCI study (Phase II Randomized, Double-Blind, Placebo-Controlled Study of Capecitabine Bevacizumab Plus Atezolizumab vs Capecitabine Bevacizumab Plus Placebo in Patients With Refractory Metastatic Colorectal Cancer) was a double-blind placebo-controlled multicenter phase 2 randomized clinical trial conducted through the Academic and Community Cancer Research United network. Study enrollment began on September 25, 2017, and ended on June 28, 2018, with data cutoff on May 4, 2020. The study was performed according to applicable regulations and guidelines governing clinical study conduct and complied with the ethical principles provided by the Declaration of Helsinki.17 The study protocol was approved by the independent ethics committee and institutional review board of all participating institutions (Supplement 1). The Mayo Clinic Cancer Center Data and Safety Monitoring Board provided safety oversight. All patients provided written informed consent, and no patient received a stipend or incentive to participate. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines for randomized clinical trials (Supplement 1).
Eligible patients were 18 years or older, had histologically confirmed CRC and clinical or histologic documentation of metastatic disease, and experienced disease progression while receiving fluoropyrimidine (eg, fluorouracil or capecitabine), oxaliplatin, irinotecan, bevacizumab, and anti–epidermal growth factor receptor antibody therapy (if the patient had a RAS wild-type tumor) or could not tolerate treatment or treatment was contraindicated. Disease progression while receiving previous therapies could have been based on clinical or radiographic progression. Patients who previously received treatment with agents targeting PD-1/PD-L1 were excluded. Additional inclusion and exclusion criteria are described in eMethods 1 in Supplement 2. Participant MSI and dMMR status were determined by local testing at individual sites. Data on self-reported race were collected to assess differences in response between races and to generally evaluate balance between the 2 treatment groups.
Procedures
Patients were recruited and enrolled by treating physicians at participating institutions and randomized (2:1) to receive capecitabine/bevacizumab plus atezolizumab (investigational group) or capecitabine/bevacizumab plus placebo (placebo group). The 2:1 randomization was chosen to promote enrollment. Treatment was assigned by computerized central randomization using the Pocock and Simon dynamic allocation procedure,18 stratified by Eastern Cooperative Oncology Group (ECOG) performance status (0 vs 1), RAS status (wild type vs variant), and enrolling institution. After treatment group was determined, the registration specialist notified the designated pharmacist at the patient’s institution, who prepared the investigational or placebo solution and maintained records on patient identity and assigned treatment. The unblinded pharmacist could not be involved in assessing any study outcomes. The treatment solution was labeled atezolizumab/placebo study drug 1 dose to ensure the contents were not discernible to the person administering the treatment. Patients and all individuals administering treatment, assessing outcomes, and collecting data were blinded to treatment assignment.
For each 21-day cycle, patients received oral capecitabine (850 or 1000 mg/m2, per investigator choice and institutional standards) twice daily on days 1 to 14, intravenous bevacizumab (7.5 mg/kg) on day 1, and either intravenous atezolizumab (1200 mg; investigational group) or placebo (placebo group) on day 1. Treatment was administered until disease progression, unacceptable toxic effects, refusal, or other reasons leading to treatment discontinuation. Capecitabine dose reductions were made (850, 680, or 545 mg/m2) if toxic effects occurred. Dose reescalation was permitted if the patient tolerated the reduced dose with grade 1 or lower toxic effects for a minimum of 2 consecutive cycles; if a dose reduction to less than 545 mg/m2 was required, capecitabine was permanently discontinued and patients could continue receiving other study treatments. No dose reductions were allowed for bevacizumab and atezolizumab. The criteria for bevacizumab and atezolizumab interruption and discontinuation are described in eMethods 2 in Supplement 2.
Outcomes
Response and progression were evaluated at least every 3 cycles by the investigator using the Response Evaluation Criteria in Solid Tumors, version 1.1.19 Among patients who discontinued treatment for reasons other than disease progression, tumor assessments were performed every 12 weeks or as clinically indicated. Adverse events (AEs) were continuously assessed. Blood and archival tumor tissue samples were collected for biomarker analysis. The primary end point was unconfirmed investigator-assessed PFS, defined as the time from randomization to first documentation of disease progression or death from any cause. Patients who were alive and without documentation of disease progression were censored at the last tumor assessment. Patients who were alive and had no postbaseline tumor measurement were censored for PFS at day 1 after randomization. A sensitivity analysis for the primary end point was performed, in which PFS was also censored at the last tumor assessment before initiation of new anticancer therapy or withdrawal from clinical follow-up. Secondary end points were ORR, OS, and toxic effects. Objective response rate was defined as the number of patients achieving (unconfirmed) partial or complete response per the Response Evaluation Criteria in Solid Tumors, version 1.1,19 criteria divided by the total number of patients in the modified intention-to-treat population. Overall survival was defined as the time from randomization to death from any cause. Patients who were alive were censored for OS at the last follow-up. Adverse events were assessed by the investigator using the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0,20 to determine possible relationship to study treatments. Immune-related AEs of grade 3 or higher that were at least possibly related to study treatments were reported.
Statistical Analysis
The goal of the study was to screen for the potential efficacy among patients in the investigational group, who received capecitabine/bevacizumab with atezolizumab.21 To ensure 120 eligible patients, and accounting for approximately 12% ineligibility and study withdrawal, we planned to randomize (2:1) a total of 135 patients to the investigational or placebo group. The primary comparison was superiority of the investigational vs placebo regimens for PFS. Median PFS in the placebo group was assumed to be approximately 2 months on the basis of published results22,23,24; 110 PFS events were required to detect a hazard ratio (HR) of 0.65 with 80% power (1-sided α = .10). Differences between groups were evaluated using an unstratified 1-sided log-rank test. The investigational group was deemed superior to the placebo group if the 1-sided log-rank P value was .10 or less. A more relaxed type I error (ie, 1-sided α = .10) was used, following the screening clinical trial design.21 A 1-sided (rather than 2-sided) type I error was used because the study focused on assessing whether the treatment regimen used in the investigational group was superior to the regimen used in the placebo group. No interim analyses of futility or efficacy were conducted. The sensitivity analysis of PFS was considered exploratory, and no multiple-testing adjustment was made. Both PFS and OS were estimated using the Kaplan-Meier method, with median values and survival rates calculated along with 95% CIs. Hazard ratios and corresponding 95% CIs were estimated using a Cox proportional hazards model. Point estimates and 95% CIs were generated for ORR, and response rates were compared between groups using a χ2 test for proportion. An exploratory subset analysis was performed among patients with known MSS and pMMR (MSS/pMMR) disease. An additional exploratory analysis was conducted to detect potential differences in treatment effects among patients with and without liver metastasis, in which the P value for interaction was calculated to quantify potential effects. Primary and secondary efficacy analyses were performed using data from the modified intention-to-treat population, which included all randomized eligible patients, regardless of the treatment received. Safety analyses included all randomized patients who received 1 or more doses of study treatment. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc).
Results
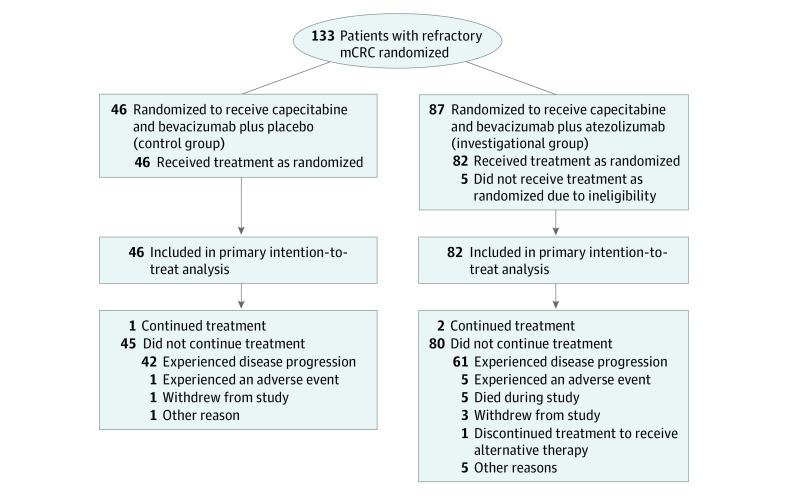
Overall, 133 patients from 9 US cancer centers or hospitals were randomized to 1 of 2 treatment groups, with 128 patients (82 in the investigational group and 46 in the placebo group) assessed for the primary end point (Figure 1). The median duration of follow-up for PFS was 20.9 months (IQR, 18.9 months to not evaluable). Demographic and disease characteristics were balanced between treatment groups (Table). The median age was 58.0 years (IQR, 51.0-65.0 years); 51 patients (39.8%) were female, and 77 (60.2%) were male. Overall, 15 patients (11.7%) self-identified as African American or Black, 8 (6.3%) as Asian, 1 (0.8%) as Pacific Islander, 101 (78.9%) as White, 1 (0.8%) as multiple races (Asian, Native Hawaiian/Pacific Islander, and White), and 2 (1.6%) as unknown race or unsure of race. A total of 60 patients (46.9%) had an ECOG performance status of 1. The colon was the primary tumor site among 84 patients (65.6%), and 74 patients (57.8%) had RAS-variant mCRC. Most patients (94 [73.4%]) had left-sided tumors. Overall, 110 patients (89.4%; 69 in the investigational group and 41 in the placebo group) had MSS/pMMR disease. There was no significant difference between groups in the percentage of doses received or the proportion of patients with at least 1 dose modification, omission, or delay for any of the study treatments (eTables 1 to 3 in Supplement 2).
Figure 1. Study Flow Diagram.
mCRC indicates metastatic colorectal cancer.
Table. Baseline Characteristics of Patients Included in Modified Intention-to-Treat Analysis.
| Characteristic | Patients, No./total No. (%) | ||
|---|---|---|---|
| Total | Investigational group (capecitabine/bevacizumab with atezolizumab) | Placebo group (capecitabine/bevacizumab with placebo) | |
| Total patients, No. | 128 | 82 | 46 |
| Age, median (IQR), y | 58.0 (51.0-65.0) | 59.0 (53.0-66.0) | 55.5 (50.0-63.0) |
| Sex | |||
| Female | 51/128 (39.8) | 35/82 (42.7) | 16/46 (34.8) |
| Male | 77/128 (60.2) | 47/82 (57.3) | 30/46 (65.2) |
| Race | |||
| African American or Black | 15/128 (11.7) | 7/82 (8.5) | 8/46 (17.4) |
| Asian | 8/128 (6.3) | 6/82 (7.3) | 2/46 (4.3) |
| Pacific Islander | 1/128 (0.8) | 1/82 (1.2) | 0 |
| White | 101/128 (78.9) | 66/82 (80.5) | 35/46 (76.1) |
| Multiple racesa | 1/128 (0.8) | 0 | 1/46 (2.2) |
| Unknown or patient unsureb | 2/128 (1.6) | 2/82 (2.4) | 0 |
| ECOG performance status | |||
| 0 | 68/128 (53.1) | 42/82 (51.2) | 26/46 (56.5) |
| 1 | 60/128 (46.9) | 40/82 (48.8) | 20/46 (43.5) |
| Primary tumor site | |||
| Colon | 84/128 (65.6) | 57/82 (69.5) | 27/46 (58.7) |
| Rectum | 44/128 (34.4) | 25/82 (30.5) | 19/46 (41.3) |
| Sidedness | |||
| Left | 94/128 (73.4) | 59/82 (72.0) | 35/46 (76.1) |
| Right or transverse | 34/128 (26.6) | 23/82 (28.0) | 11/46 (23.9) |
| RAS status | |||
| Variant | 74/128 (57.8) | 50/82 (61.0) | 24/46 (52.2) |
| Wild type | 54/128 (42.2) | 32/82 (39.0) | 22/46 (47.8) |
| MSI status | |||
| Missing | 5/128 (3.9) | 4/82 (4.9) | 1/46 (2.2) |
| MSS/pMMR | 110/123 (89.4) | 69/78 (88.5) | 41/45 (91.1) |
| MSI high | 9/123 (7.3) | 6/78 (7.7) | 3/45 (6.7) |
| dMMR | 4/123 (3.3) | 3/78 (3.8) | 1/45 (2.2) |
Abbreviations: dMMR, deficient mismatch repair; ECOG, Eastern Cooperative Oncology Group; MSI, microsatellite instability; MSS, microsatellite stable; pMMR, proficient mismatch repair.
Patient self-identified as multiple races (Asian, Native Hawaiian/Pacific Islander, and White).
Patient did not know or was unsure of race.
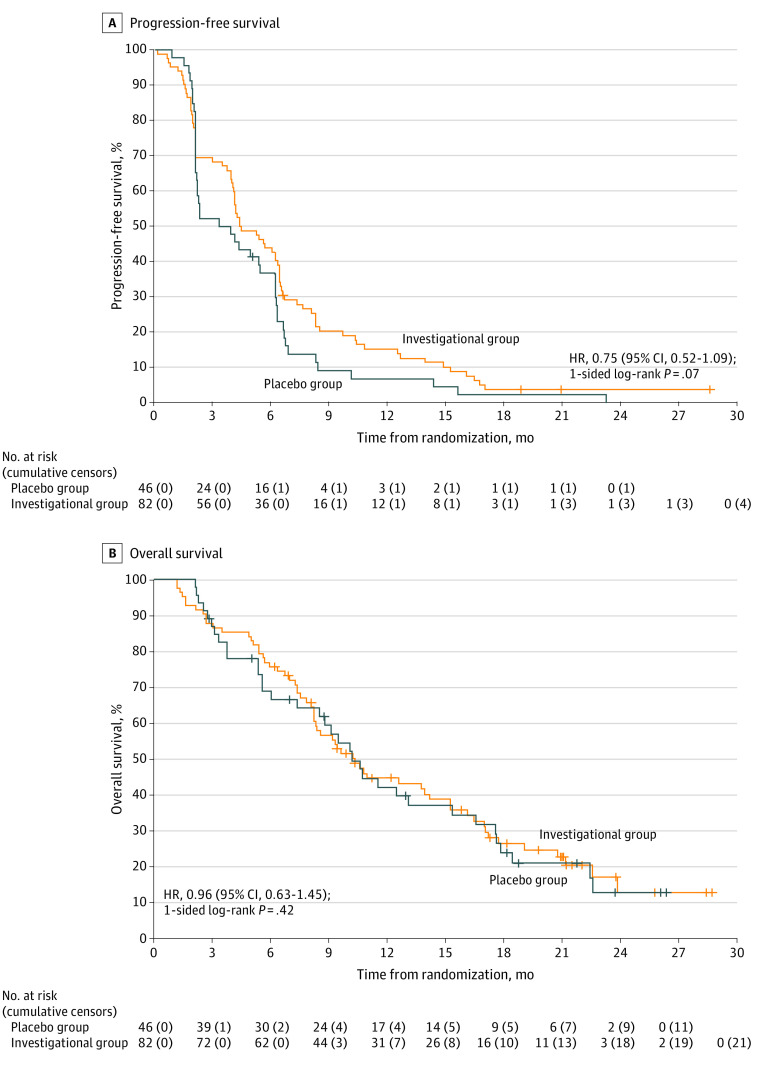
In the primary analysis, among 123 PFS events, median PFS in the investigational vs placebo groups was 4.4 months (95% CI, 4.1-6.4 months) vs 3.6 months (95% CI, 2.2-6.2 months; HR, 0.75 [95% CI, 0.52-1.09]; 1-sided log-rank P = .07) (Figure 2A). The 1-sided log-rank P value of .07 was statistically significant and lower than the α value of .10 prespecified in the statistical design, which demonstrated that the study met its primary end point. The 12-month PFS estimate was 15.2% (95% CI, 9.1%-25.6%) in the investigational group vs 6.9% (95% CI, 2.3%-20.5%) in the placebo group. Among patients with MSS/pMMR tumors, the HR for PFS was 0.66 (95% CI, 0.44-0.99) (eFigure 1A and 1B in Supplement 2). In the sensitivity analysis of the primary end point, in which PFS was censored before initiation of new anticancer therapy and withdrawal from clinical follow-up, median PFS was similar to that of the primary analysis (4.4 months [95% CI, 4.1-6.4 months] in the investigational group vs 3.9 months [95% CI, 2.1-6.2 months] in the placebo group). However, in the sensitivity analysis, the 1-sided log-rank P value of .15 (HR, 0.82; 95% CI, 0.56-1.20) did not cross the efficacy boundary (eFigure 2 in Supplement 2).
Figure 2. Progression-Free and Overall Survival.
Patients in the investigational group received capecitabine and bevacizumab with atezolizumab, and patients in the placebo group received capecitabine and bevacizumab with placebo. Plus sign (+) indicates time of censor. A, Among 82 patients in the investigational group, 78 progression-free survival (PFS) events occurred (median, 4.4 months [95% CI, 4.1-6.4 months]; 6-month estimate, 43.9% [95% CI, 34.4%-56.1%]; 12-month estimate, 15.2% [95% CI, 9.1%-25.6%]). Among 46 patients in the placebo group, 45 PFS events occurred (median, 3.6 months [95% CI, 2.2-6.2 months]; 6-month estimate, 36.7% [95% CI, 25.1%-53.8%]; 12-month estimate, 6.9% [95% CI, 2.3%-20.5%]). B, Among 82 patients in the investigational group, 61 overall survival (OS) events occurred (median, 10.3 months [95% CI, 8.3-15.2 months]; 6-month estimate, 75.6% [95% CI, 66.9%-85.5%]; 12-month estimate, 44.5% [95% CI, 34.7%-57.1%]). Among 46 patients in the placebo group, 35 OS events occurred (median, 10.2 months [95% CI, 8.5-16.6 months]; 6-month estimate, 68.8% [95% CI, 56.5%-83.8%]; 12-month estimate, 42.0% [95% CI, 29.4%-59.9%]). HR indicates hazard ratio.
Median OS was similar in the investigational vs placebo groups (10.3 months [95% CI, 8.3-15.2 months] vs 10.2 months [95% CI, 8.5-16.6 months]; HR, 0.96 [95% CI, 0.63-1.45]; 1-sided log-rank P = .42) (Figure 2B); 61 OS events occurred in the investigational group, and 35 occurred in the placebo group. The 12-month survival estimates were 44.5% (95% CI, 34.7%-57.1%) and 42.0% (95% CI, 29.4%-59.9%) among patients who received the investigational and placebo regimens, respectively.
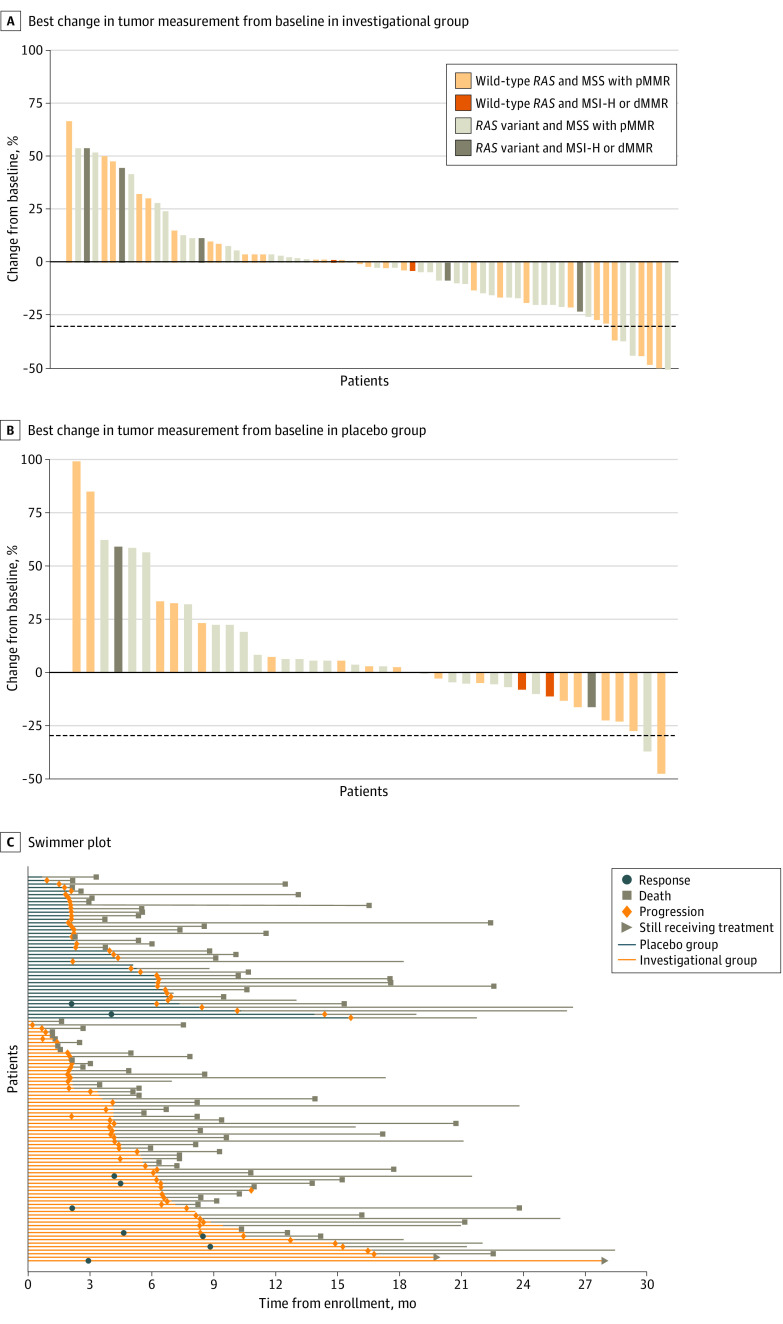
The ORR was 7 of 82 patients (8.5%; 95% CI, 3.5%-16.8%) in the investigational group vs 2 of 46 patients (4.4%; 95% CI, 0.5%-14.8%; P = .37) in the placebo group. Among those with MSS/pMMR tumors, the ORR was 7 of 69 patients (10.1%; 95% CI, 4.2%-19.8%) in the investigational group compared with 2 of 41 patients (4.9%; 95% CI, 0.6%-16.5%; P = .33) in the placebo group. All 9 patients who achieved a response in either the investigational or placebo group had MSS/pMMR disease; there were no responses among patients with MSI-H and dMMR (MSI-H/dMMR) tumors. Microsatellite instability–high disease can be associated with high rates of BRAF variants and aggressive biological features. However, most patients with MSI-H (11 of 13 [84.6%]) in the study had BRAF wild-type tumors (eTable 4 in Supplement 2). Of the 9 patients with MSI-H/dMMR in the investigational group, 2 patients (22.2%) had BRAF variants. The median duration of response was 5.6 months (95% CI, 2.0 months to not evaluable) among the 7 responding patients from the investigational group and 7.2 months (95% CI, 4.1 months to not evaluable) among the 2 responding patients from the placebo group. The best percentage change in tumor measurement from baseline per patient is shown in Figure 3A and B. The response and progression outcomes among patients with MSS/pMMR disease are shown in Figure 3C.
Figure 3. Best Percentage Change in Tumor Measurement From Baseline and Swimmer Plot.
A and B, Dashed line indicates 30% reduction in tumor measurement compared with baseline. C, Swimmer plot shows response and progression among patients with microsatellite stable (MSS) and proficient mismatch repair (pMMR) disease. Patients in the investigational group received capecitabine and bevacizumab with atezolizumab, and patients in the placebo group received capecitabine and bevacizumab with placebo. The tan lines (after blue or orange) indicate the follow-up period. dMMR indicates deficient mismatch repair; and MSI-H, microsatellite instability high.
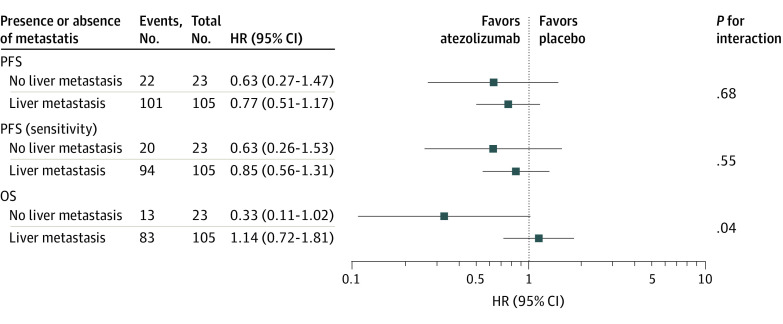
An exploratory analysis showed that the proportion of patients with liver metastasis was similar between the groups (eTable 5 in Supplement 2). In a post hoc analysis, patients in the investigational group without liver metastasis had a higher response rate (3 of 13 individuals [23.1%]) compared with those with liver metastasis (4 of 69 individuals [5.8%]; P = .04) (eTable 6 in Supplement 2). The benefit of atezolizumab for OS among patients without liver metastasis (HR, 0.33; 95% CI, 0.11-1.02) was significantly greater than the benefit of atezolizumab among patients with liver metastasis (HR, 1.14; 95% CI, 0.72-1.81; P = .04 for interaction). The benefit of atezolizumab for PFS (primary and sensitivity analyses) among patients without liver metastasis was also greater than that for patients with liver metastasis (primary analysis: HR, 0.63 [95% CI, 0.27-1.47] vs 0.77 [95% CI, 0.51-1.17], respectively; P = .68 for interaction; sensitivity analysis: HR, 0.63 [95% CI, 0.26-1.53] vs 0.85 [95% CI, 0.56-1.31]; P = .55 for interaction); however, the difference was not statistically significant in either analysis (Figure 4).
Figure 4. Survival End Points of Investigational vs Placebo Groups by Presence and Absence of Liver Metastasis.
HR indicates hazard ratio; OS, overall survival; and PFS, progression-free survival.
In the safety analysis population, 80 patients experienced grade 3 or higher AEs (53 patients [61.6%] in the investigational group and 27 patients [58.7%] in the placebo group), regardless of potential relationship to study treatments. The most frequently reported grade 3 or higher AEs in the investigational vs placebo groups were hypertension (grade 3: 9 patients [10.5%] vs 5 patients [10.9%]), abdominal pain (grade 3: 7 patients [8.1%] vs 5 patients [10.9%]), diarrhea (grade 3: 8 patients [9.3%] vs 2 patients [4.3%]), hand-foot syndrome (grade 3: 6 patients [7.0%] vs 4 patients [8.7%]), and fatigue (grade 3: 6 patients [7.0%] vs 2 patients [4.3%]). The most common grade 3 or higher AEs considered to be at least possibly related to study treatment in the investigational vs placebo groups were hypertension (grade 3: 6 patients [7.0%] vs 2 patients [4.3%]), diarrhea (grade 3: 6 patients [7.4%] vs 2 patients [4.3%]), hand-foot syndrome (grade 3: 6 patients [7.0%] vs 2 patients [4.3%]), and fatigue (grade 3: 3 patients [3.5%] vs 1 patient [2.2%]). Grade 3 or higher immune-related AEs that were considered to be at least possibly related to study treatment are summarized in eTable 7 in Supplement 2.
One treatment-related death (classified as death not otherwise specified) occurred in the investigational group. An additional 4 patients in the investigational group died while receiving active treatment; none of those deaths were related to study treatment. A total of 17 grade 5 AEs were reported (12 in the investigational group and 5 in the placebo group).
Discussion
In this randomized clinical trial, the addition of atezolizumab to capecitabine/bevacizumab met the threshold for statistical significance for the primary PFS end point; however, the improvement in PFS was of limited clinical benefit. The rationale for a capecitabine/bevacizumab placebo group was to offer some palliative chemotherapy to a population with highly refractory mCRC and to assess the added activity of anti–PD-1/PD-L1 therapy vs chemotherapy with anti-VEGF therapy for the treatment of mCRC. Patients in the placebo group received capecitabine/bevacizumab and performed better than patients in placebo groups of other clinical trials of refractory mCRC, in which true placebo was used.22,23,24 The PFS of 4.4 months in the investigational group was longer than the PFS of 2.0 months previously reported with the use of other agents approved for the treatment of refractory mCRC.22,23,24
This study predominantly included patients with MSS/pMMR mCRC (89.4%). Seven responses were observed in the investigational group (ORR, 8.5%), and 2 were observed in the placebo group (ORR, 4.4%); notably, all of those who responded to treatment had MSS disease. To our knowledge, this study is the second phase 2 randomized clinical trial in which PD-1/PD-L1 inhibition yielded responses in patients with MSS tumors.23 No responses were observed among patients with MSI-H/dMMR mCRC in either group. The number of patients with MSI-H/dMMR tumors was small. Microsatellite instability status was self-reported by each site and was not validated before study participation. Although anti–PD-1 therapy is effective for the treatment of MSI-H/dMMR mCRC, anti–PD-L1 therapy may not be as effective. We considered whether a higher rate of BRAF variants could account for the lack of responses among patients with MSI-H/dMMR tumors receiving atezolizumab. Of the 9 patients with MSI-H/dMMR tumors in the investigational group, only 2 (22.2%) had BRAF variants, suggesting that the lack of response was not the result of more aggressive biological features. Correlative analyses to confirm site-reported MSI status will be important to further understand this finding.
Dual inhibition of the VEGF and PD-1/PD-L1 axes has yielded clinical activity in various solid tumors,13,25 including mCRC,26 with prolonged survival outcomes among patients with hepatocellular carcinoma, nonsquamous non–small cell lung cancer, and renal cell carcinoma.10,11,12,13 The activity of this combination in multiple diseases suggests a broad role for VEGF in tumor immunologic response.27 Although small, the improvement in PFS in our study may have derived from inhibition of VEGF-mediated immune evasion in combination with anti–PD-1/PD-L1 therapy. The benefit of dual inhibition of the VEGF and PD-1/PD-L1 axes in mCRC may depend on the line of therapy. Although the addition of atezolizumab to fluoropyrimidine and bevacizumab in the maintenance setting did not improve PFS or OS, the refractory setting may result in a higher neoantigen load and improved sensitivity to checkpoint inhibition.16,28,29
Understanding factors associated with response may identify subsets of patients who may benefit from this approach. Patients with MSS/pMMR mCRC and those without liver metastasis may benefit more from dual inhibition of the VEGF and PD-1/PD-L1 axes,30 possibly related to differences in the immune microenvironment in liver metastases.31 Our study demonstrated that patients without liver metastasis who were receiving investigational treatment had a higher ORR (23.1%) compared with those with liver metastasis (5.8%) and derived greater OS benefit from the investigational treatment than patients with liver metastasis. Future studies could focus on dual inhibition of the VEGF and PD-1/PD-L1 axes in patients with MSS/pMMR mCRC who do not have a history of liver metastasis. Tumor mutational burden has emerged as a biomarker of response to anti–PD-1/PD-L1 therapy,32 but there may be differences in archival tissue vs plasma tumor mutational burden.33 Comprehensive immune profiling being conducted in collaboration with National Cancer Institute–funded Cancer Immune Monitoring and Analysis Center laboratories may provide biological insight into differences between those who respond and do not respond to treatment.
Strengths and Limitations
This study has strengths. These strengths include its randomized, double-blinded, multicenter design as well as its sample size and statistical power to detect a difference in PFS.
This study also has limitations. The study lacked independent or central image review, confirmatory scans, and the use of a standardized methodologic approach for determining MSI status. Long-term follow-up was limited by the data cutoff date, and the Immune Response Evaluation Criteria in Solid Tumors approach34 was not used.
Conclusions
This randomized clinical trial met its primary PFS end point; however, the addition of atezolizumab to capecitabine/bevacizumab did not provide a clinically meaningful increase in PFS among the overall population of patients with refractory mCRC. In post hoc analyses, some clinical activity was observed among patients with MSS/pMMR mCRC, particularly those without liver metastasis.
Trial Protocol
eMethods 1. Additional Inclusion and Exclusion Criteria
eMethods 2. Criteria for Bevacizumab and Atezolizumab Interruption and Discontinuation
eTable 1. Dosing Information for Atezolizumab and Placebo
eTable 2. Dosing Information for Bevacizumab
eTable 3. Dosing Information for Capecitabine
eTable 4. BRAF Variation Status of Primary Analysis Population
eTable 5. Sites of Metastatic Disease (mITT)
eTable 6. Response Rates in the Investigational Arm by Sites of Metastatic Disease
eTable 7. Immune-Related Adverse Events Grade 3 or Higher Considered at Least Possibly Related to Treatment
eFigure 1. PFS of Atezolizumab vs Placebo by MSS Status
eFigure 2. Sensitivity Analysis of PFS
Data Sharing Statement
References
- 1.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. doi: 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair–deficient or microsatellite instability–high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182-1191. doi: 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair–deficient/microsatellite instability–high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773-779. doi: 10.1200/JCO.2017.76.9901 [DOI] [PubMed] [Google Scholar]
- 4.André T, Shiu KK, Kim TW, et al. ; KEYNOTE-177 Investigators . Pembrolizumab in microsatellite-instability–high advanced colorectal cancer. N Engl J Med. 2020;383(23):2207-2218. doi: 10.1056/NEJMoa2017699 [DOI] [PubMed] [Google Scholar]
- 5.Eng C, Kim TW, Bendell J, et al. ; IMblaze370 Investigators . Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019;20(6):849-861. doi: 10.1016/S1470-2045(19)30027-0 [DOI] [PubMed] [Google Scholar]
- 6.Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118(1):9-16. doi: 10.1038/bjc.2017.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249-257. doi: 10.1038/35025220 [DOI] [PubMed] [Google Scholar]
- 8.Rahma OE, Hodi FS. The intersection between tumor angiogenesis and immune suppression. Clin Cancer Res. 2019;25(18):5449-5457. doi: 10.1158/1078-0432.CCR-18-1543 [DOI] [PubMed] [Google Scholar]
- 9.Osada T, Chong G, Tansik R, et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother. 2008;57(8):1115-1124. doi: 10.1007/s00262-007-0441-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finn RS, Qin S, Ikeda M, et al. ; IMbrave150 Investigators . Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi: 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 11.Socinski MA, Jotte RM, Cappuzzo F, et al. ; IMpower150 Study Group . Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301. doi: 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 12.Rini BI, Powles T, Atkins MB, et al. ; IMmotion151 Study Group . Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393(10189):2404-2415. doi: 10.1016/S0140-6736(19)30723-8 [DOI] [PubMed] [Google Scholar]
- 13.Rini BI, Plimack ER, Stus V, et al. ; KEYNOTE-426 Investigators . Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127. doi: 10.1056/NEJMoa1816714 [DOI] [PubMed] [Google Scholar]
- 14.Bendell JC, Powderly JD, Lieu CH, et al. Safety and efficacy of MPDL3280A (anti-PDL1) in combination with bevacizumab (bev) and/or FOLFOX in patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol. 2015;33(3)(suppl):704. doi: 10.1200/jco.2015.33.3_suppl.704 [DOI] [Google Scholar]
- 15.Hochster HS, Bendell JC, Cleary JM, et al. Efficacy and safety of atezolizumab (atezo) and bevacizumab (bev) in a phase Ib study of microsatellite instability (MSI)–high metastatic colorectal cancer (mCRC). J Clin Oncol. 2017;35 (4)(suppl):673. doi: 10.1200/JCO.2017.35.4_suppl.673 [DOI] [Google Scholar]
- 16.Grothey A, Tabernero J, Arnold D, et al. Fluoropyrimidine (FP) + bevacizumab (BEV) + atezolizumab vs FP/BEV in BRAFwt metastatic colorectal cancer (mCRC): findings from cohort 2 of MODUL—a multicentre, randomized trial of biomarker-driven maintenance treatment following first-line induction therapy. Ann Oncol. 2018;29(suppl 8):viii714-viii715. [Google Scholar]
- 17.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 18.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103-115. doi: 10.2307/2529712 [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New Response Evaluation Criteria in Solid Tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute, National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. US Department of Health and Human Services; 2009. Accessed July 1, 2020. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf
- 21.Rubinstein LV, Korn EL, Freidlin B, Hunsberger S, Ivy SP, Smith MA. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005;23(28):7199-7206. doi: 10.1200/JCO.2005.01.149 [DOI] [PubMed] [Google Scholar]
- 22.Mayer RJ, Van Cutsem E, Falcone A, et al. ; RECOURSE Study Group . Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909-1919. doi: 10.1056/NEJMoa1414325 [DOI] [PubMed] [Google Scholar]
- 23.Chen EX, Jonker DJ, Loree JM, et al. Effect of combined immune checkpoint inhibition vs best supportive care alone in patients with advanced colorectal cancer: the Canadian Cancer Trials Group CO.26 study. JAMA Oncol. 2020;6(6):831-838. doi: 10.1001/jamaoncol.2020.0910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grothey A, Van Cutsem E, Sobrero A, et al. ; CORRECT Study Group . Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303-312. doi: 10.1016/S0140-6736(12)61900-X [DOI] [PubMed] [Google Scholar]
- 25.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103-1115. doi: 10.1056/NEJMoa1816047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukuoka S, Hara H, Takahashi N, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38(18):2053-2061. doi: 10.1200/JCO.19.03296 [DOI] [PubMed] [Google Scholar]
- 27.Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med. 2020;52(9):1475-1485. doi: 10.1038/s12276-020-00500-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown JS, Sundar R, Lopez J. Combining DNA damaging therapeutics with immunotherapy: more haste, less speed. Br J Cancer. 2018;118(3):312-324. doi: 10.1038/bjc.2017.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Donnell T, Christie EL, Ahuja A, et al. Chemotherapy weakly contributes to predicted neoantigen expression in ovarian cancer. BMC Cancer. 2018;18(1):87. doi: 10.1186/s12885-017-3825-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang C, Chevalier D, Saluja J, Sandhu J, Lau C, Fakih M. Regorafenib and nivolumab or pembrolizumab combination and circulating tumor DNA response assessment in refractory microsatellite stable colorectal cancer. Oncologist. 2020;25(8):e1188-e1194. doi: 10.1634/theoncologist.2020-0161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou SN, Pan WT, Pan MX, et al. Comparison of immune microenvironment between colon and liver metastatic tissue in colon cancer patients with liver metastasis. Dig Dis Sci. 2021;66(2):474-482. doi: 10.1007/s10620-020-06203-8 [DOI] [PubMed] [Google Scholar]
- 32.Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353-1365. doi: 10.1016/S1470-2045(20)30445-9 [DOI] [PubMed] [Google Scholar]
- 33.Loree JM, Topham JT, Kennecke HF, et al. Tissue and plasma tumor mutation burden (TMB) as predictive biomarkers in the CO.26 trial of durvalumab + tremelimumab (D+T) versus best supportive care (BSC) in metastatic colorectal cancer (mCRC). J Clin Oncol. 2021;39(3)(suppl): 61. doi: 10.1200/JCO.2021.39.3_suppl.61 [DOI] [Google Scholar]
- 34.Seymour L, Bogaerts J, Perrone A; RECIST Working Group . iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3): e143-e152. doi: 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods 1. Additional Inclusion and Exclusion Criteria
eMethods 2. Criteria for Bevacizumab and Atezolizumab Interruption and Discontinuation
eTable 1. Dosing Information for Atezolizumab and Placebo
eTable 2. Dosing Information for Bevacizumab
eTable 3. Dosing Information for Capecitabine
eTable 4. BRAF Variation Status of Primary Analysis Population
eTable 5. Sites of Metastatic Disease (mITT)
eTable 6. Response Rates in the Investigational Arm by Sites of Metastatic Disease
eTable 7. Immune-Related Adverse Events Grade 3 or Higher Considered at Least Possibly Related to Treatment
eFigure 1. PFS of Atezolizumab vs Placebo by MSS Status
eFigure 2. Sensitivity Analysis of PFS
Data Sharing Statement