Abstract
Objectives
To evaluate the effectiveness of remdesivir in the early stage of nonsevere COVID-19. Although several randomized controlled trials have compared the effectiveness of remdesivir with that of a placebo, there is limited evidence regarding its effect in the early stage of nonsevere COVID-19 cases.
Methods
We evaluated the effectiveness of remdesivir in the early stage of nonsevere COVID-19 using the COVID-19 Registry Japan, a nationwide registry of hospitalized patients with COVID-19 in Japan. Two regimens (“start remdesivir” therapy within 4 days from admission versus no remdesivir during hospitalization) among patients without the need for supplementary oxygen therapy were compared by a 3-step processing (cloning, censoring, and weighting) method. The primary outcome was a supplementary oxygen requirement during hospitalization. Secondary outcomes were 30-day in-hospital mortality and the risk of invasive mechanical ventilation or extracorporeal membrane oxygenation (IMV/ECMO). The data of 12,487 cases met our inclusion criteria. The “start remdesivir” regimen showed a lower risk of supplementary oxygen requirement (hazard ratio [HR]: 0.850, 95% confidence interval [CI]: 0.798–0.906, p value < 0.001). Both 30-day in-hospital mortality and risk of IMV/ECMO introduction were not significantly different between the 2 regimens (HRs: 1.04 and 0.983, 95% CI: 0.980–1.09 and 0.906–1.07, p values: 0.210 and 0.678, respectively).
Conclusions
Remdesivir might reduce the risk of oxygen requirement during hospitalization in the early stage of COVID-19; however, it had no positive effect on the clinical outcome and reduction in IMV/ECMO requirement.
Keywords: COVID-19, Remdesivir, Inverse probability treatment weighting
Introduction
As in other parts of the world, the number of patients with COVID-19 is increasing in Japan, with 5,274,596 cases and 24,604 deaths reported from January 14, 2020, to March 5, 2022 (Ministry of Health, Labour and Welfare, 2022). In addition to the treatment of the hyperinflammatory state and coagulopathy, antiviral medication is one of the important components of COVID-19 treatment (Cevik et al., 2020). Among the antiviral medications for SARS-CoV-2, only remdesivir was approved in Japan on May 7, 2020 (Pharmaceuticals and Medical Devices, 2020).
Several randomized controlled trials (RCTs) have compared the effectiveness of remdesivir with that of a placebo. In an RCT in China that enrolled hospitalized patients with COVID-19 pneumonia with hypoxia, no statistically significant clinical benefits were observed (Wang et al., 2020). A multinational RCT (ACTT-1) conducted in Europe, the United States, and Asia, including Japan (Saito et al., 2021), confirmed that remdesivir shortened the time to recovery in hospitalized patients with COVID-19 with pneumonia (Beigel et al., 2020). However, in the subgroup analysis, no reduction in the time to recovery was observed in patients who were intubated or on extracorporeal membrane oxygenation (ECMO) at the time of the drug administration. In addition, there was no reduction in time to recovery in the subgroup of patients with no oxygen requirement (RR for time to recovery 1.29 (95% CI: 0.91-1.83)). Although the recovery rate improvement observed among patients enrolled from Asia was similar to that among the overall population, ethnically Asian patients did not show such treatment benefit. The multinational SOLIDARITY Trial, organized by the World Health Organization, demonstrated no survival benefit for remdesivir in hospitalized patients with COVID-19 (WHO SOLIDARITY Trial Consortium et al., 2020). The trial enrolled 61% of patients from Asia and Africa, but no patients from Japan were enrolled. In another RCT conducted in the United States, Europe, and Asia, including 16%–19% Asians, no statistically significant difference was observed between the 10-day remdesivir group and the standard treatment group (Spinner et al., 2020). These data indicate conflicting results regarding remdesivir's clinical effectiveness, and currently, recommendations in the guidelines of remdesivir use against COVID-19 are inconsistent, and its optimal role remains uncertain (World Health Organization).
Although the effectiveness of remdesivir against severe COVID-19 cases has been already examined in several studies, its effectiveness against nonsevere cases or cases in the early stage of disease has not yet been evaluated. In the study of Spinner et al. (Spinner et al., 2020), patients with COVID-19 pneumonia were targeted with preserved room-air oxygen saturation. However, the interpretation of the results of this trial is limited by the inconsistent evidence of the treatment regimens.
We conducted this study to evaluate the effectiveness of remdesivir in nonsevere patients with COVID-19 in the early stage of disease, especially the stage before the initiation of supplementary oxygen therapy and other pharmaceutical treatment.
Methods
Study population and data
We used the data of patients derived from COVIREGI-JP (Matsunaga et al., 2020). The inclusion criteria are both (1) a positive SARS-CoV-2 test (polymerase chain reaction [PCR] test and/or rapid antigen test) and (2) inpatient treatment at a healthcare facility. SARS-CoV-2 testing is based on the notification criteria of the Infectious Diseases Law (Ministry of Health and Labour, 2022). Patients who refused to participate in the study by opting out were excluded.
We had modified a case report form of the International Severe Acute Respiratory and Emerging Infection Consortium (2020). Study data were collected and managed using REDCap (Research Electronic Data Capture), Associates and Clinicians (JCRAC) data center of the National Center for Global Health and Medicine.
We used data from cases that had entered all the following major items as of April 30, 2021 (i.e., frozen data as of April 30, 2021), for this study, similar to the previous report (Matsunaga et al., 2020): basic information at admission (demographics and epidemiological characteristics), comorbidities, signs, and symptoms at the time of admission (including conditions at admission), the outcome at discharge, supportive care during hospitalization, history of drug administration during hospitalization, and complications during hospitalization.
Study design
Eligibility for analysis set
Among all patients registered as cases of COVIREGI-JP, we excluded non-Japanese patients and patients aged <18 years to evaluate the effectiveness of remdesivir in the Japanese adult cohort. We also excluded patients with severe diseases who had already been initiated on supplementary oxygen therapy during admission and/or admitted more than 4 days before the day of symptom onset to evaluate the effectiveness of remdesivir in the early stages of treatment.
Endpoints, treatment strategies of interest, and follow-up
The primary outcome was oxygen therapy requirement during 30 days of admission. The secondary outcomes were 30-day fatality risk and risk of IMV or ECMO. We compared the following treatment regimens: Regimen 1, start remdesivir therapy within 4 days from the day of admission for at least 3 days and at the most 15 days without the combination of systemic steroids and some immunosuppressive agents or antivirals (tocilizumab, baricitinib, and favipiravir), and Regimen 2, not using remdesivir, other immunosuppressive agents or antivirals (tocilizumab, baricitinib, and favipiravir), and systemic steroids during their admission. Other supportive treatments were allowed in both regimens. The indication of remdesivir as defined by the Ministry of Health, Labor and Welfare are as follows: i) oxygen saturation ≤ 94% (ambient air), ii) requirement of supplementary oxygen/IMV/ECMO, and iii) pneumonia because of SARS-CoV-2 (added from January 2021).
Each patient was followed-up until 30 admission days, an event of interest (initiation of oxygen therapy for primary analysis, death, or initiation of IMV/ECMO within 30 admission days for secondary analysis), and discharge, whichever came first. Furthermore, we required both regimens to withhold the initiation of supplementary oxygen therapy for 4 days from admission to evaluate the effectiveness of remdesivir among patients without the need for intensive therapy at admission. We excluded patients who were initiated on oxygen therapy within 4 days from admission; the possible time-related biases associated with such exclusion after admission (the start of follow-up) (Hernán et al., 2016; Suissa and Dell'Aniello, 2020) were addressed by the novel statistical approach described in the next section.
Statistical analysis
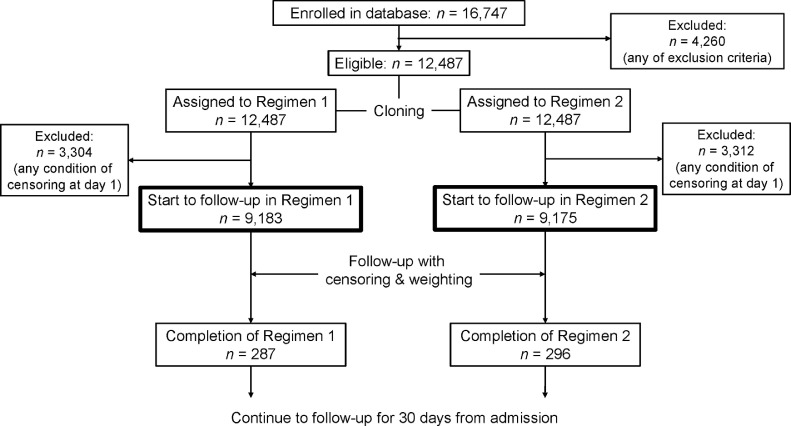
To compare the previously mentioned treatment regimens from time-varying remdesivir treatment data in an unbiased manner, we used the ‘3-step’ method (cloning, censoring, and weighting) (Hernán, 2018). First, we prepared clones (or data copies) of patients to assign them to the 2 regimens on a person-day basis. We assigned each person to the treatment regimens at admission at which the ‘eligibility’ of enrollment was judged. We assigned patients treated with remdesivir at Day 1 to the ‘start remdesivir’ regimen arm and other patients to both regimens. Simultaneously assigning a patient to both arms is equivalent to having 2 clones of that patient in the dataset, with each copy assigned to a different arm. The cloning process was performed only once before censoring.
Second, we artificially censored the clones if they deviated from their assigned regimen during the follow-up period. For instance, consider a patient who was initiated on remdesivir between days 1 and 4; his/her clone assigned to Regimen 2 (‘no remdesivir’) was censored at that time, but the clone assigned to Regimen 1 (‘start remdesivir’) was followed-up after that. Conversely, for a patient not initiated on remdesivir at Day 5, his/her clone assigned to Regimen 1 was censored at Day 5, but the clone assigned to Regimen 2 was followed-up thereafter. In addition, clones were censored at any time when the following conditions were met: (1) supported by supplementary oxygen before 4 days from admission, (2) treated with systemic steroids, (3) treated with tocilizumab, (4) treated with baricitinib, (5) treated with favipiravir, (6) duration of remdesivir treatment shorter than 3 days (patients were censored when they discontinued remdesivir before the 3 days elapsed from treatment initiation), and (7) duration of remdesivir treatment longer than 15 days (patients were censored at 15 days if they continued using remdesivir). Moreover, when we compared the primary outcome (supplementary oxygen requirement), patients were excluded from the risk set on the next day of the beginning day of oxygen administration. Similarly, when we compared the secondary outcomes, IMV/ECMO introduction and death within 30 days from admission were the signs of censoring. Discharged patients were censored from the next day of discharge. We set the duration of observation as 30 days, and all patients were censored after 30 days had elapsed since their admission. The process of cloning and censoring is shown in Figure 1 .
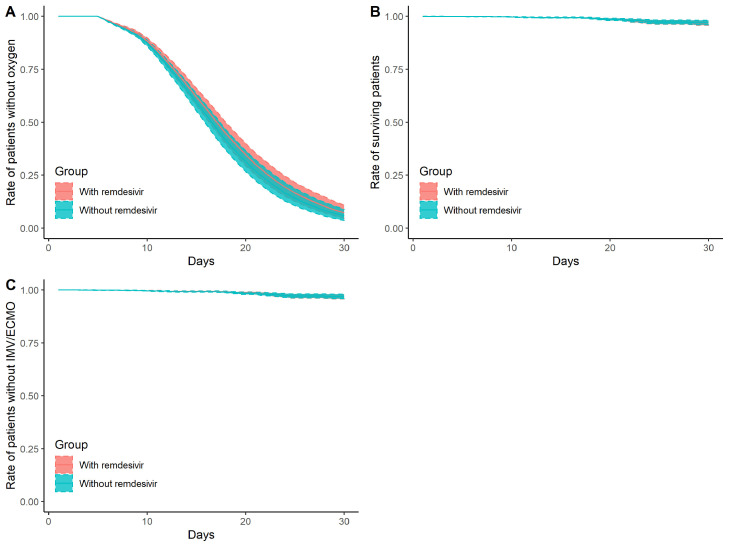
Figure 2.
Daily cumulative probability of presenting primary/secondary outcomes
Panel A: daily cumulative probability of not being supported by oxygen
Panel B: daily probability of survival
Panel C: daily cumulative probability of not being supported by invasive mechanical ventilation (IMV)/extracorporeal membrane oxygenation (ECMO)
Red ribbons represent Regimen 1 (treated with remdesivir), and blue ribbons represent Regimen 2 (treated without remdesivir). Shaded zones represent pointwise 95% confidence intervals by bootstrapping.
Figure 1.
Flow diagram of the cloning process
Third, to eliminate selection bias because of the previously mentioned artificial censoring, we used the inverse probability of censoring/discharge weights (Robins and Finkelstein, 2000). The weights of each person-day were calculated using pooled logistic regression models for being censored or discharged, such as age, sex, cardiovascular diseases, chronic respiratory diseases, diabetes mellitus, severe renal diseases (serum creatinine level: ≥3 mg/dl), or dialysis, hypertension, hyperlipidemia, obesity diagnosed by physicians, solid tumor, days from symptom onset to admission, use of corticosteroids, use of anticoagulants (time-independent variables), and National Early Warning Score (NEWS, time-dependent variable) (The National Health Service England, 2017.). The models were fitted separately according to regimens and follow-up days. The weights were stabilized according to the regimen-day-specific probability without covariate and were multiplied until that day of the follow-up. Especially, we had only intermittent data about the clinical course of the patient. In this study, we had information on patients on days 1, 4, 8, 15, 22, and 29. For example, a patient's record indicating administered oxygen at Day 8 implies that oxygen support for that patient began between days 5 and 8 and the exact day is not available. We used NEWS at Day 1 as that of Day 1; NEWS at Day 4 as that of days 2, 3, and 4; NEWS at Day 8 as that of days 5, 6, 7, and 8; and NEWS at Day 15 as that of days 9, 10, 11, 12, 13, 14, and 15, same as NEWS at days 22 and 29. These possible confounders were selected for their potential association with the outcome of interest-based on clinical knowledge and previous studies (Cunningham et al., 2020; Lighter et al., 2020; Petrilli et al., 2020; Tartof et al., 2020; Williamson et al., 2020; Wu and McGoogan, 2020).
Finally, the discrete-time hazard ratio of primary and secondary outcomes between the 2 regimens was estimated using weighted pooled logistic regression included primary, secondary, and tertiary terms of days as covariates. As each patient has multiple lines in the dataset (each day, each regimen of the same patient until censored), we used cluster-robust standard errors regarding each patient as a cluster to estimate 95% confidence intervals (CIs). We also estimated cumulative incidence rates under the 2 regimens by multiplying the weighted probabilities of no-event using the Kaplan-Meier method. The pointwise 95% CIs at each day were based on 2.5 and 97.5 percentiles of 1,000 bootstrap estimates. All statistical analyses were conducted using R, version 4.1.2 (R Core Team, 2018).
Results
The data of 12,487 of 16,747 cases met our inclusion criteria Table 1. lists the basic characteristics of the included cases. A total of 824 patients were treated with remdesivir, and the treatment duration depended on each facility and physician's decision. The duration of remdesivir treatment was 5 days in 485 cases (58.9%). The 10-day regimen was completed in 105 cases (12.7%). A total of 115 patients (14.0%) were administered remdesivir for <5 days, and 88 (10.7%) were administered between 6 and 9 days. A total of 27 patients (3.3%) were administered for >10 days. Inappropriate duration (smaller than zero) was recorded for 3 patients, and then they were excluded from the final analysis. Patients in the case group were older, more frequently male, and more severe and fatal.
Table 1.
Basic characteristics of patients who met the inclusion criteria
| Patients initiated on remdesivir (n = 824) | Patients without remdesivir (n = 11,663) | Total (n = 12,487) | |
|---|---|---|---|
| Age (years) | 68 [56–79] | 51 [34–71] | 52 [35–71] |
| Male | 532 (64.6%) | 6,176 (53.0%) | 6,708 (53.7%) |
| Cardiovascular disease | 57 (6.9%) | 547 (4.7%) | 604 (4.8%) |
| Respiratory disease | 34 (4.1%) | 268 (2.3%) | 302 (2.4%) |
| Diabetes mellitus | 225 (27.3%) | 1,415 (12.1%) | 1,640 (13.1%) |
| Severe renal disease or dialysis | 19 (2.3%) | 200 (1.7%) | 219 (1.8%) |
| Hypertension | 367 (44.5%) | 2,845 (24.4%) | 3,212 (25.7%) |
| Obesity | 90 (10.9%) | 809 (6.9%) | 899 (7.2%) |
| Charlson Comorbidity Index | 1 [0–2] | 0 [0–1] | 0 [0–1] |
| NEWS at Day 1 | 1 [0–2] | 1 [0–2] | 1 [0–2] |
| NEWS at Day 4 | 2 [1–4] | 1 [0–2] | 1 [0–2] |
| NEWS at Day 8 | 2 [1–4] | 1 [0–2] | 1 [0–2] |
| NEWS at Day 15 | 2 [1–4] | 1 [0–2] | 1 [0–3] |
| NEWS at Day 22 | 3 [1–5] | 1 [0–3] | 1 [0–3] |
| NEWS at Day 29 | 11 [9–13] | 9 [9–11] | 9 [9–11] |
| Fatal cases | 69 (8.4%) | 285 (2.4%) | 354 (2.8%) |
| Oxygen administration during hospitalization* | 559 (67.8%) | 1,784 (15.3%) | 2,343 (18.8%) |
| IMV/ECMO during hospitalization | 48 (5.8%) | 98 (0.8%) | 146 (1.2%) |
| Days from symptom onset to hospitalization | 3 [1–4] | 3 [1–4] | 3 [1–4] |
| Use of systemic steroids | 666 (80.8%) | 1,840 (15.8%) | 2,506 (20.1%) |
| Use of favipiravir | 264 (32.0%) | 2,926 (25.1%) | 3,190 (25.5%) |
| Use of tocilizumab | 63 (7.7%) | 100 (0.9%) | 163 (1.3%) |
| Use of baricitinib | 0 (0%) | 0 (0%) | 0 (0%) |
| Days from onset to remdesivir administration | 6 [4–9] | NA | NA |
| Days from admission to remdesivir administration | 5 [3–10] | NA | NA |
| Duration of remdesivir administration 5 days | 485 (58.9%) | NA | NA |
Numbers in brackets represent percentage and interquartile range.
NA = not available; NEWS = national early warning score; IMV/ECMO = invasive mechanical ventilation/extracorporeal membrane oxygenation.
Indication for supplementary oxygen was judged by each physician.
Table 2 lists the characteristics of the patients at the beginning of the observation after weighted by inverse probability of censoring or discharge.
Table 2.
Characteristics of the patients after weighted by inverse probability of censoring/discharge at the beginning of the observation
| Regimen 1 (treated with remdesivir) | Regimen 2 (treated without remdesivir) | Standardized mean difference | |
|---|---|---|---|
| Number | 9,183 | 9,175 | |
| Age (years) | 50.3 (21.5) | 50.2 (21.5) | 0.004 |
| Male | 52.3% | 52.5% | 0.002 |
| Cardiovascular disease | 4.1% | 4.1% | <0.001 |
| Respiratory disease | 2.0% | 2.0% | 0.003 |
| Diabetes mellitus | 10.4% | 10.4% | <0.001 |
| Severe renal disease or dialysis | 1.3% | 1.3% | 0.002 |
| Hypertension | 21.3% | 21.1% | 0.006 |
| Obesity | 6.5% | 6.5% | 0.003 |
| Charlson Comorbidity Index | 0.45 (0.95) | 0.45 (0.95) | 0.001 |
| NEWS at Day 1 | 1.02 (1.17) | 1.01 (1.17) | 0.002 |
| NEWS at Day 4 | 1.18 (1.34) | 1.18 (1.34) | 0.001 |
| NEWS at Day 8 | 1.28 (1.58) | 1.28 (1.57) | 0.001 |
| NEWS at Day 15 | 1.62 (2.01) | 1.63 (2.02) | 0.004 |
| NEWS at Day 22 | 1.88 (2.39) | 1.87 (2.38) | 0.005 |
| NEWS at Day 29 | 10.11 (1.92) | 10.13 [1.96] | 0.011 |
| Fatal cases | 1.7% | 1.7% | <0.001 |
| Oxygen administration during hospitalization* | 9.4% | 9.4% | 0.001 |
| IMV/ECMO during hospitalization | 0.5% | 0.5% | 0.001 |
| Days from symptom onset to hospitalization | 2.62 (1.60) | 2.62 (1.60) | <0.001 |
| Use of systemic steroids | 9.6% | 9.5% | 0.005 |
| Use of favipiravir | 9.4% | 9.4% | 0.002 |
| Use of tocilizumab | 0.4% | 0.4% | <0.001 |
| Use of baricitinib | 0 (0%) | 0 (0%) | <0.001 |
Regimen 1: treated with remdesivir.
Regimen 2: treated without remdesivir.
Continuous variables are presented mean (standard deviation). Categorical variables are presented in percentage.
IMV/ECMO = invasive mechanical ventilation/extracorporeal membrane oxygenation; NEWS = national early warning score.
Regimen 1 (treated with remdesivir within 4 days of admission) showed a lower risk (adjusted HR: 0.850, 95% CIs: 0.798–0.906, p < 0.001) of supplementary oxygen requirement than Regimen 2 (treated without remdesivir). However, the 30-day fatality risk and risk of IMV/ECMO introduction were not different between the 2 groups (adjusted HR: 1.04 [95% CIs: 0.981–1.09] and 0.983 [95% CIs: 0.906–1.07], p values: 0.217 and 0.678, respectively) Table 3. lists the details of primary and secondary outcomes.
Table 3.
Results of pooled logistic regression analysis on the effect of remdesivir on primary and secondary outcomes
| Person-days | Event | Weighted event rate (per 1,000 person-day) | Hazard ratio | 95% CI | P value | |
|---|---|---|---|---|---|---|
| Oxygen requirement | ||||||
| Regimen 1 | 85,129 | 324 | 3.81 | 0.850 | 0.798–0.906 | <0.001 |
| Regimen 2 | 85,606 | 379 | 4.43 | 1 | Reference | |
| 30-day fatality risk | ||||||
| Regimen 1 | 85,129 | 33 | 0.388 | 1.04 | 0.981–1.09 | 0.2170 |
| Regimen 2 | 85,606 | 33 | 0.385 | 1 | Reference | |
| IMV/ECMO* | ||||||
| Regimen 1 | 85,129 | 42 | 0.493 | 0.983 | 0.906–1.07 | 0.678 |
| Regimen 2 | 85,606 | 44 | 0.514 | 1 | Reference |
Regimen 1: treated with remdesivir.
Regimen 2: treated without remdesivir.
CI = confidence interval; IMV/ECMO = invasive mechanical ventilation/extracorporeal membrane oxygenation.
Figure 1 shows the daily cumulative probability of presenting primary and secondary outcomes. The distribution of inverse probability weights is shown in Supplementary Figure S1.
Regarding the safety of remdesivir treatment, 92 of 824 (11.2%) cases reported adverse events (Table 4 ), of whom 24 (26.1%) were considered as having probable relevance to remdesivir. Although 66 patients (71.7%) continued remdesivir treatment despite adverse events, the remaining 26 (28.3%) suspended their treatment. A total of 45 patients (48.9%) had liver dysfunction or liver enzyme elevation, 10 (10.9%) reported renal dysfunction, 3 (3.3%) had nausea/vomiting, and 4 (4.3%) showed rash. No patient had sequelae because of adverse events.
Table 4.
Adverse events during remdesivir treatment
| Severity | Number of cases | Probable relevance to remdesivir | Cessation of remdesivir | Sequelae |
|---|---|---|---|---|
| Mild Liver dysfunction (38) Renal failure (14) Other (17) |
69 (8.4%*) | 16 (23.2%) | 17 (24.6%) | 0 (0%) |
| Moderate Liver dysfunction (5) Renal failure (4) Rash (3) Other (8) |
20 (2.4%*) | 7 (35.0%) | 8 (40.0%) | 0 (0%) |
| Serious Renal failure (2) Neutropenia (1) |
3 (0.4%*) | 1 (33.3%) | 1 (33.3%) | 0 (0%) |
*Denominators are the total number of cases treated with remdesivir (n = 824)
Mild: adverse events need no treatment or presented no symptom.
Moderate: adverse events need non-invasive treatment.
Serious: important adverse events need invasive treatment.
Discussion
Our study showed that remdesivir administration in the early stage of disease might reduce the supplementary oxygen requirement during hospitalization. However, it did not reduce fatality risk and risk of IMV/ECMO requirement in hospitalized patients with COVID-19. These results concerning fatality and IMV/ECMO are compatible with a previous study (WHO Solidarity Trial Consortium et al., 2020) and support that remdesivir is not an essential drug for COVID-19-specific treatment, as suggested by the latest clinical guideline (CDC, 2020; Ministry of Health and Labour, 2021; World Health Organization, 2021). Similarly, this study demonstrated the possible benefit of using remdesivir in the early stage of the disease. Our results suggest that among nonsevere hospitalized patients (i.e., patients without oxygen requirement), early initiation of remdesivir is beneficial.
However, further discussion would be desirable because our analysis demonstrated that the drug of interest did not improve the final prognosis of the disease. Although the risk of severe adverse events because of remdesivir appears to be low, we can consider it an unnecessary risk if the drug does not improve the outcome. Conversely, a substantial benefit can be obtained by reducing the burden on healthcare facilities if it prevents patients with COVID-19 from the need for supplementary oxygen therapy.
Furthermore, we must note that the healthcare system in Japan allowed us to hospitalize even nonsevere patients. For instance, Japanese indications for hospitalization are quite different from those of other countries (Ministry of Health and Labour, 2021.; National Institutes of Health, 2021; World Health Organization, 2021); therefore, it is difficult to apply our results directly to different settings. In addition, the hospitalization criteria in Japan have been changing over the COVID-19 pandemic period (Yamada et al.). Initially, the indication of remdesivir in Japan was limited to severe COVID-19 cases (Ministry of Health and Labour, 2020). Remdesivir was approved in Japan in May 2020 by the fast-track approval followed by the United States Food and Drug Administration Emergency Use Authorization (Coronavirus COVID-19 Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment, 2020). At that time, the indication of remdesivir was limited to severe cases whose oxygen saturation was ≤94% (ambient air) and who required supplementary oxygen or IMV/ECMO. In January 2021, the Ministry of Health, Labor, and Welfare in Japan extended its indication to “patients who have pneumonia because of SARS-CoV-2 infection.” Consequently, the number of mild or moderate cases administered remdesivir increased recently, and it enabled us to analyze the effectiveness of remdesivir in the early stage of the disease. Further evaluation in other healthcare settings will be one of the future challenges.
Our study has some limitations. The most important is that it is not an RCT but a retrospective cohort study. Certainly, we sincerely attempted to adjust various factors that affect clinical outcomes; however, our method does not enable us to adjust all the numerous confounding factors (Rosenbaum and Rubin, 1983). Although our method enables us to adjust time-dependent factors and immortal time bias (Hernán, 2018; Hernán et al., 2016), we could not include time-dependent variables other than NEWS. Moreover, as our data are based on a registry system, it is difficult to interpret several items. For instance, “fatality” in this study implies that a patient died during his/her 30-day observation period, i.e., during hospitalization. Even if a patient died after he/she was discharged, we labeled this patient as one who survived. The cause of death is also unavailable from the registry data, and when a fatal case has a serious comorbidity such as cancer, we are not aware of the disease that caused the death of the patient. Furthermore, COVIREGI-JP does not collect information about the daily clinical status of each patient. The adverse events of remdesivir were reported based on researchers’ decisions and thus might be underreported. Nevertheless, our data at least appropriately adjust the time-independent characteristics of cases associated with clinical outcomes (e.g., age, comorbidity, etc.), and an important time-dependent factor deeply associated with their clinical course and outcome (i.e., NEWS); hence, we believe that the results were reliable.
For the evaluation of drug safety, we must consider that our registry contains limited data about adverse events, especially the relation between symptoms and drugs. However, considering only some adverse events and no reported sequelae, we can consider remdesivir to be a relatively safe drug.
Conclusions
Remdesivir might reduce supplementary oxygen requirement during hospitalization. However, it showed no positive effect on the clinical outcome.
Funding
This study was supported by the Health and Labor Sciences Research Grant, “Research on Emerging and Re-emerging Infectious Diseases and Immunization” Program (19HA1003).
Ethics
This study was approved by the NCGM ethics review (NCGM-G-003494-0). Information regarding opting out of our study is available on the registry website.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all the participating facilities for caring for COVID-19 cases and cooperation in data entry.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.02.039.
Appendix. Supplementary materials
References
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19 — final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Coronavirus Disease 2019 (COVID-19) Centers for Disease Control and Prevention. 2020 https://www.cdc.gov/coronavirus/2019-ncov/index.html (accessed August 12, 2021) [Google Scholar]
- Cevik M, Kuppalli K, Kindrachuk J, Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020;371:m3862. doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- Cunningham JW, Vaduganathan M, Claggett BL, Jering KS, Bhatt AS, Rosenthal N, et al. Clinical outcomes in young US adults hospitalized with COVID-19. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernán MA. How to estimate the effect of treatment duration on survival outcomes using observational data. BMJ. 2018;360:k182. doi: 10.1136/bmj.k182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernán MA, Sauer BC, Hernández-Díaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016;79:70–75. doi: 10.1016/j.jclinepi.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020;71:896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga N, Hayakawa K, Terada M, Ohtsu H, Asai Y, Tsuzuki S, et al. Clinical epidemiology of hospitalized patients with COVID-19 in Japan: report of the COVID-19 REGISTRY JAPAN. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharmaceuticals and Medical Devices Agency. Remdesivir, 2020. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/62504A3 (accessed January 27, 2021).
- International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC). COVID-19 Clinical Research Resources, 2020. https://isaric.tghn.org/covid-19-clinical-research-resources/ (accessed January 18, 2021).
- Ministry of Health, Labour and Welfare. The number of patients and other information about COVID-19 in Japan, 2022. https://www.mhlw.go.jp/stf/covid-19/kokunainohasseijoukyou.html (accessed March 5, 2022).
- Ministry of Health, Labour and Welfare. Notification of physicians and veterinarians under the Infectious Diseases Act, 2021. https://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou11/01-shitei-01.html (accessed January 18, 2021a).
- Ministry of Health, Labour and Welfare. Indication of remdesivir, 2020. https://www.mhlw.go.jp/stf/seisakunitsuite/newpage_00051.html (accessed January 28, 2021c).
- Ministry of Health, Labour and Welfare. Clinical Guideline for COVID-19, Aug 2021. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000121431_00111.html. (accessed August 12, 2021b).
- Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. Information on COVID-19 treatment, prevention and research. COVID-19 Treatment Guidelines, Jan 2021. https://www.covid19treatmentguidelines.nih.gov/ (accessed January 27, 2021).
- Food and Drug Administration . FDA; 2020. Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment (accessed January 31, 2021) [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2018. R: A language and environment for statistical computing. [Google Scholar]
- Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56:779–788. doi: 10.1111/j.0006-341X.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- Saito S, Hayakawa K, Mikami A, Izumi S, Funazaki H, Ashida S, et al. Investigator initiated clinical trial of remdesivir for the treatment of COVID-19 in Japan. GHM. 2021;3:62–66. doi: 10.35772/ghm.2020.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suissa S, Dell'Aniello S. Time-related biases in pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2020;29:1101–1110. doi: 10.1002/pds.5083. [DOI] [PubMed] [Google Scholar]
- Tartof SY, Qian L, Hong V, Wei R, Nadjafi RF, Fischer H, et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173:773–781. doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Health Service England. National Early Warning Score (NEWS). https://www.england.nhs.uk/ourwork/clinical-policy/sepsis/nationalearlywarningscore/ (accessed January 28, 2021).
- Wang Yeming, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Solidarity Trial Consortium, Pan H, Peto R, Henao-Restrepo A-M, Preziosi M-P, Sathiyamoorthy V, et al. Repurposed antiviral drugs for COVID-19 - Interim WHO solidarity trial results. N Engl J Med. 2020 doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, McGoogan JM. Characteristics of and Important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Therapeutics and COVID-19: living guideline, 2021. https://www.who.int/publications-detail-redirect/therapeutics-and-covid-19-living-guideline (accessed January 27, 2021). [PubMed]
- Yamada G, Hayakawa K, Matsunaga N, Terada M, Suzuki S, Asai Y, et al. Predicting respiratory failure for COVID-19 patients in Japan: a simple clinical score for evaluating the need for hospitalization. Epidemiology & Infection undefined/ed:1–38. doi: 10.1017/S0950268821001837. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.