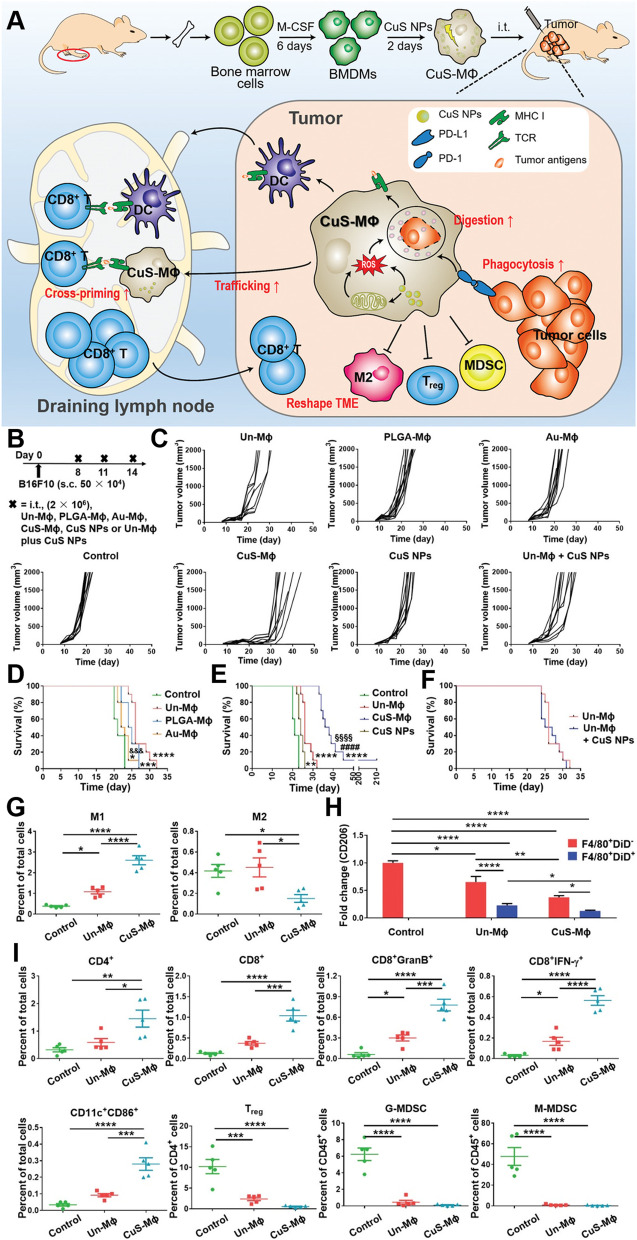
Fig. 9.
A Schematic illustration of redirecting macrophages by CuS NPs for adoptive transfer therapy of solid tumor. B-I Adoptive transfer of CuS-MΦ for enhanced activity against murine melanoma. B Treatment regimen. i.t., Intratumoral injection. C Individual B16F10 tumor growth curves following the treatment with Un-MΦ, PLGA-MΦ, Au-MΦ, CuS-MΦ, CuS NPs alone, or Un-MΦ plus CuS NPs (n = 10). Control, mice without treatment. For CuS NPs alone group or Un-MΦ plus CuS NPs group, injection dose of CuS NPs was 0.3 μg of Cu, which was equivalent to that of 2 × 106 of CuS-MΦ. D-F Kaplan-Meier survival curves of selected compared groups, log-rank analysis (n = 10). G Quantitative analysis of classic macrophages (M1, CD11b + F4/80 + CD206–) versus alternative macrophages (M2, CD11b + F4/80 + CD206+) in tumor on day 20. One-way ANOVA with Tukey’s post-test (n = 5). H The expression of CD206 in either the transferred (F4/80 + DiD+) or tumor-associated (F4/80 + DiD–) macrophages analyzed on the 3rd day after i.t. transfer of the DiD-labeled CuS-MΦ or Un-MΦ to mice bearing B16F10 tumor, normalized by Control group. One-way ANOVA with Tukey’s post-test (n = 5–6). I The population of immune cells in the tumor at day 20 including CD4+ T cells, CD8+ T cells, granzyme B-positive CD8+ T cells (CD8 + GranB+), CTLs (CD8 + IFN-γ+), activated DCs (CD11c + CD86+), Treg cells (CD4 + CD25 + Foxp3+), as well as CD11b + Gr-1+ myeloid-derived suppressor cell (MDSC) subsets including CD11b + Gr-1high granulocytic MDSCs (G-MDSCs) and CD11b + Gr-1int monocytic MDSCs (M-MDSCs). One-way ANOVA with Tukey’s post-test (n = 5). Data are expressed as mean ± s.e.m. [157]