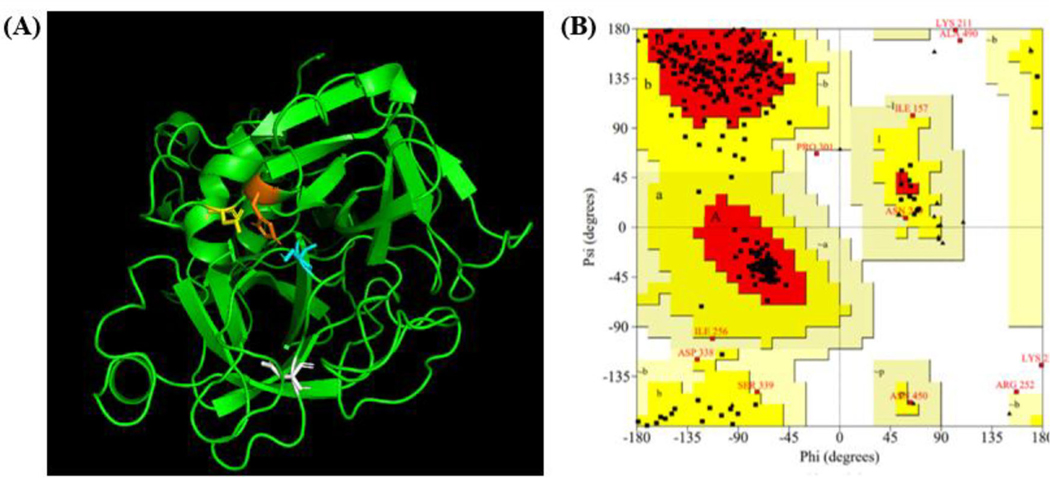
Figure 2.
Constructed structure of TMPRSS2. (A) The active site of the catalytic triad consists of His296 (orange), Asp345 (yellow), and Ser441 (blue). Substrate binding residue Asp435 in white. The figure was generated with PyMOL. (B) The Ramachandran plot, generated by PROCHECK (Laskowski et al., 1996; Laskowski et al., 1993), shows a vast majority of residues lying within the most favored (red) and allowed (Δark yellow) regions. Six residues lie within the generously allowed region (pale yellow) and four lie within the disallowed region (white).