Figure 2.
HLA-E/Nsp13232–240 complexes fail to bind to CD94/NKG2A
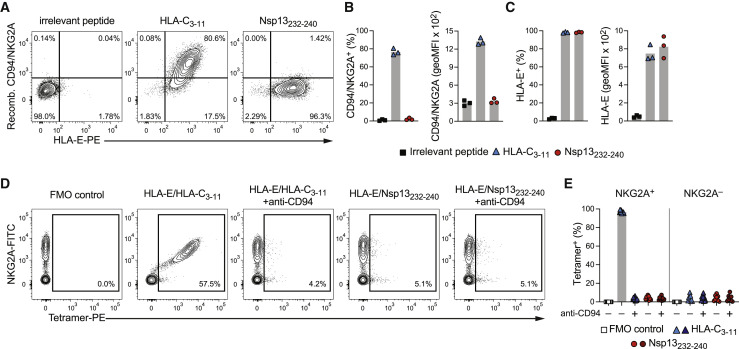
(A–C) HLA-E stabilization and binding of recombinant (recomb.) CD94/NKG2A protein to K562/HLA-E after peptide pulsing at 300 μM overnight in serum-free medium. (A) Representative binding of recombinant CD94/NKG2A as determined by flow cytometry after pulsing with the indicated peptides. (B) Summaries of CD94/NKG2A binding. Left: frequency of K562/HLA-E cells positive for CD94/NKG2A. Right: degree of CD94/NKG2A bound by K562/HLA-E cells presented as geoMFI (n = 3 independent experiments). (C) Summaries of HLA-E stabilization in the same experiments. Left: frequency of K562/HLA-E cells on which HLA-E is stabilized at the cell surface. Right: degree of HLA-E surface stabilization presented as geoMFI (n = 3 independent experiments).
(D and E) Binding of HLA-E tetramers to primary CD56dim NKG2C− NK cells. (D) Representative binding of tetramers refolded with the indicated peptides in the absence or presence of blocking anti-CD94 antibodies. (E) Summary of tetramer binding to CD56dim NKG2C− NKG2A+ and CD56dim NKG2C− NKG2A− NK cells without or with CD94 blockade (n = 9 donors in 3 independent experiments).
Data are mean and individual data points (B, C, and E). FMO, fluorescence minus one.