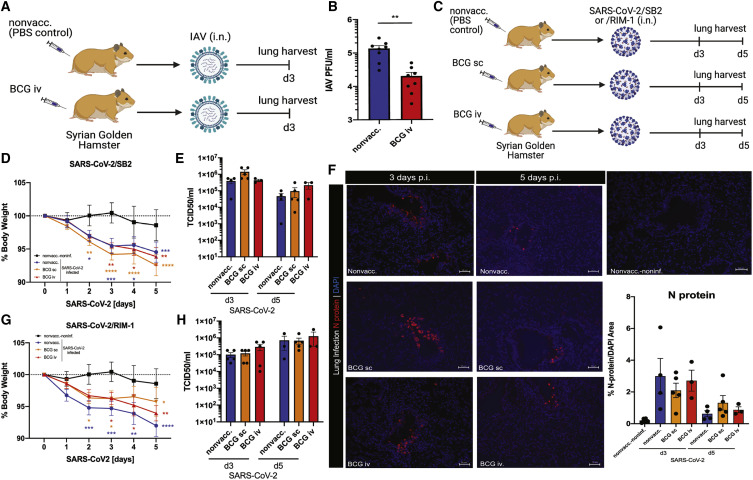
Figure 2.
BCG vaccination protects Syrian golden hamsters against IAV, but not SARS-CoV-2 infection
(A) Experimental model of BCG vaccination and i.n. infection with IAV-H3N2 in Syrian golden hamsters.
(B) Lung viral load in BCG-i.v.-vaccinated and nonvaccinated control Syrian golden hamsters at day 3 post i.n. infection with 1 × 105 PFU IAV-H3N2, n = 8/group.
(C) Experimental model of BCG vaccination and i.n. infection with SARS-CoV-2/SB2 or/RIM-1 in Syrian golden hamsters.
(D–F) Morbidity (n = 7–10/group) (D), lung viral load by TCID50/mL measurement (n = 3–5/group) (E), and representative images and quantification of viral N protein staining in the lungs of golden hamsters infected with 1 × 105 PFU SARS-CoV-2/SB2 (n = 3–5/group) (F).
(G and H) Morbidity (n = 7–10/group) (G) and lung viral load determined by TCID50/mL assay (n = 3–5/group) (H) in BCG-vaccinated and control Syrian golden hamsters at 3 and 5 days post i.n. infection with 1 × 105 PFU SARS-CoV-2/RIM-1.
Stars in morbidity curves (D and G) indicate significant weight loss compared with day 0. Data are displayed as mean ± SEM. ∗p < 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001 (t test and two-way ANOVA). See also Figure S2, Table S2.