Figure 4. Two acidic motifs in SNX1 mediate binding to SNX27.
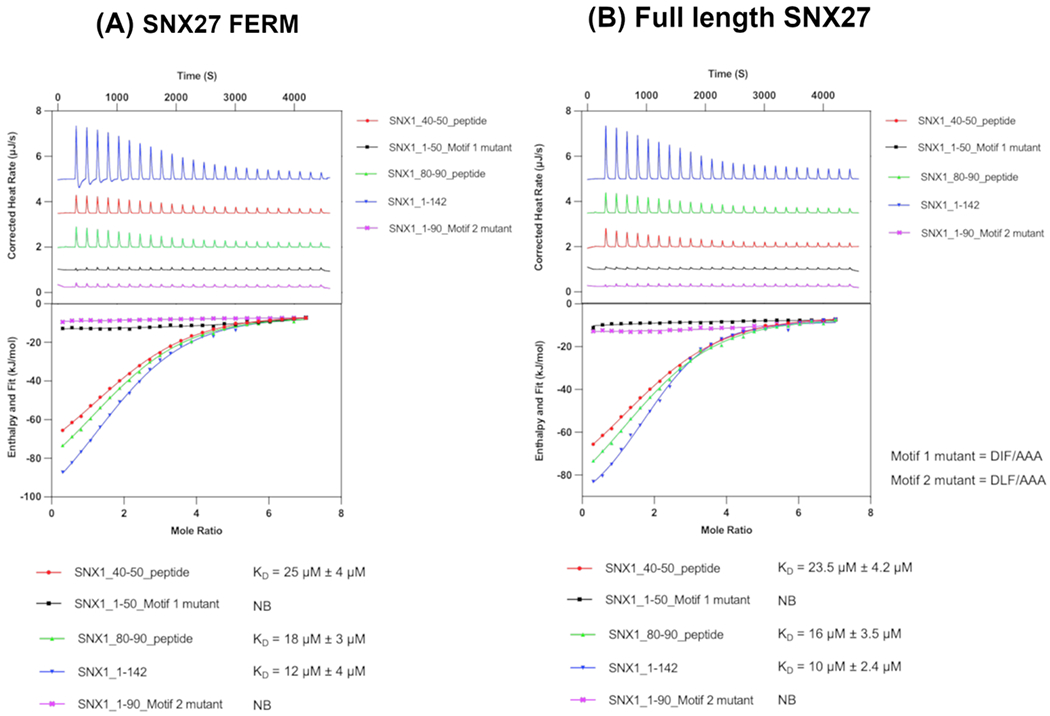
(A) Representative isotherms of SNX27 FERM domain with the SNX1 N-terminus (residues 1-142) and SNX1 peptides representing the first (SNX1 residues 40-50) or second (SNX1 residues 80-90) acidic DxF motifs. SNX1 N-terminus binds SNX27 FERM domain with a low micromolar KD (12 μM). Mutation of either DxF sequence to AAA abrogates measurable binding (denoted “NB”) to SNX27 FERM in the calorimeter. ITC experiments were conducted three times to generate reported standard deviation values. (B) Representative isotherms of full-length SNX27 with the SNX1 N-terminus (residues 1-142) and SNX1 peptides representing the first (SNX1 residues 40-50) or second (SNX1 residues 80-90) acidic DxF motifs. As with the FERM domain, mutation of the DxF sequence to AAA abrogates measurable binding. Together, these data further suggest the FERM domain mediates binding to SNX1. Both motifs bind SNX27, and the second DLF motif appears to play the predominant role.
