Abstract
Background
Temporary transvenous pacing (TP) has been associated with an increased risk of cardiac implantable electronic device (CIED) infections, but there is little data to document this in contemporary populations.
Objective
To investigate the impact of active fixation TP on rate of CIED infections in a nationwide cohort of Danish patients.
Methods
We identified all patients who underwent a first-time CIED implantation between 2009 and 2017. Patients were categorized according to TP status at implantation and followed for 1 year. The primary outcome was local or systemic CIED infection resulting in device system removal. The secondary outcomes were systemic CIED infections and hospitalization for infective endocarditis (IE).
Results
We included a total of 40,601 CIED patients. A total of 2952 were treated with active fixation TP. The primary outcome was met in 246 patients. Risk of CIED infection at 1 year was 0.61% for patients not treated with TP and 0.65% for patients who were, HR of 1.28 (95% CI 0.80–2.05) and adjusted HR 0.85 (95% CI 0.51–1.42). More systemic CIED infections and IE hospitalizations occurred in TP patients; however, these differences did not persist after confounder adjustment. Cumulative mortality at 1 year was 16.8% in patients with TP vs 8.4% in patients without.
Conclusion
Active fixation TP was not associated with a higher rate of CIED infections. Patients treated with TP had higher mortality, more systemic CIED infections, and more IE hospitalizations within first year of implantation. Most was attributable to an accumulation of risk factors for infection among TP patients.
Keywords: Cardiac implantable electronic device, Complications, Epidemiology, Infection, Infective endocarditis, Pacemaker, Temporary transvenous pacing
Key Findings.
-
▪
Active fixation temporary pacing (TP) was not associated with a higher rate of cardiac implantable electronic device (CIED) infections.
-
▪
Patients treated with active fixation TP had more risk factors for infection.
-
▪
Patients treated with active fixation TP had higher mortality, more systemic CIED infections, and more infective endocarditis hospitalizations within first year of implantation.
-
▪
Although active fixation TP did not appear to independently increase risk of infection, our study reinforced the fact that patients selected for TP are at high risk of systemic infections, and any permanent CIED implantation in patients previously treated with TP should urge special care and considerations.
Introduction
Temporary transvenous pacing (TP) can provide a therapeutic bridge for patients with life-threatening bradyarrhythmias in exceptional cases when circumstances do not allow for immediate permanent pacemaker (PM) implantation—or when the pacing requirement is considered transient. Guidelines recommend minimal use of TP owing to reports of high complication rates.1, 2, 3 These include an increased risk of lead dislodgement,4,5 cardiac perforation,4,6 thrombosis,7,8 and infection.9,10
Over the past decades, ultrasound-guided TP insertion and new pacing wire technologies have emerged and encouraged changes to clinical practice.11, 12, 13, 14 Preprocedural antibiotic prophylaxis as well as optimized management of anticoagulant and antiplatelet therapy have become standard of care in prevention of cardiac implantable electronic device (CIED) infections.2 Even so, much of our knowledge about complications after TP derives from older studies or small, single-center experiences and case series, leaving us with little large-scale data to document device-specific complications to TP in unselected, contemporary populations. The aim of this study was to investigate the impact of active fixation TP on risk of CIED infection in a nationwide cohort of consecutive Danish patients.
Methods
Study population
We included all patients aged ≥18 years who underwent a first-time transvenous CIED implantation in Denmark between August 2009 and December 2017. Patients were identified using the Danish Pacemaker and ICD Register (DPIR), and were followed for 1 year from time of first CIED implantation. We divided patients into 2 groups: those treated with TP prior to the first CIED implantation (TP group), and those who were not (non-TP group). TP was in all cases achieved using a standard active fixation lead connected to the ventricular lead port of a permanent PM. All patients received preprocedural antibiotics—prior to TP initiation and before the permanent CIED implantation. Use of peri- and postprocedural antibiotics, intrapocket hemostatics and saline irrigation, and postoperative pressure dressing was not considered standard of care, but was available at the discretion of the implanter. An antibacterial envelope only became available for Danish patients in 2015, but was used only rarely, mainly for patients undergoing device reoperations, not eligible for this study. This study was approved by the Central Denmark Region (1-16-02-199-18) and the DPIR Steering Committee. According to Danish law, register-based studies do not require ethics committee approval or informed consent from patients.
Data sources
For this study, we used data from 3 nationwide Danish registries: the DPIR, the National Patient Registry (DNPR), and the National Prescription Registry (NPR). The DPIR is a national clinical quality database in which detailed information about all Danish CIED implantations has been collected prospectively since 1982. The NPR holds information about redeemed prescriptions in Denmark,15 and the DNPR holds complete records on Danish hospital admissions. Reporting to DNPR is a prerequisite for financial reimbursement for hospitals. Individual-level linkage between registries was possible using the civil registration number, a unique personal identifier issued to every Danish resident. Cardiovascular diagnosis, procedure, and surgery codes in DNPR have previously been validated for use in research.16,17
Study outcomes and variable definitions
The primary outcome was CIED infection, defined as any infection resulting in device system removal. The secondary outcomes were systemic CIED infections and hospitalization for infective endocarditis (IE). All-cause mortality was assessed for both groups. IE was included to account for infections that did not trigger system removal. We defined this outcome as a primary or secondary admission for IE lasting ≥2 weeks. If a patient died during an IE hospitalization, they were included as a case. Transfer(s) between departments within 24 hours was considered 1 admission. This definition of IE using DNPR data has been validated to have a positive predictive value of 90%.18 Outcome data were obtained from DPIR (CIED infection) and DNPR (IE hospitalization) and indexed on date of device system removal (DPIR) or, in case of IE, on day of hospital admission (DNPR). DPIR was also used to identify patients treated with TP prior to first CIED implantation. Age at implantation was subdivided into 4 groups: <60, 60–69, 70-79, and >80 years. Center type was defined as university or non–university center. Device type was categorized as PM, implantable cardioverter-defibrillator (ICD), cardiac resynchronization therapy pacemaker (CRT-P), or CRT-defibrillator (CRT-D). Immunosuppressant therapy included any prescription medication known to reduce resistance to infections. ICD-8/-10, NOMESCO, and ATC codes are listed in Supplemental Table 1.
Statistical analyses
Baseline characteristics were tabulated for patients and summarized as frequencies with percentages. One-year cumulative incidence curves and proportions were computed for infection, taking into account the competing risks of death and heart transplantation. We applied a Cox proportional hazard model stratified by device type to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for the association between TP and infection. The proportionality assumption was assessed using log-log plots and was found to be acceptable. Patients were followed from time of first CIED implantation, and censoring occurred at 1 year, death, heart transplantation, emigration, CIED reoperation, or end of study (April 15, 2018). In the multivariable Cox regression analyses, adjustment was made for the following covariates; age, sex, oral immunosuppressant therapy, connective tissue disease, heart failure, insulin-treated diabetes, renal insufficiency, dialysis, anticoagulant/antiplatelet therapy, prior endocarditis, valve replacement surgery, center type, recent central venous catheter, and C-reactive protein. Supplementary analyses on all outcomes were conducted using a propensity score (PS)-based method. The PS is the conditional probability of treatment. When the outcome is rare, PS-based methods are able to balance multiple covariates between treatment groups without risk of overfitting. We modeled the PS using logistic regression including all covariates listed in Supplemental Table 2.19 Based on the PS, we generated stabilized inverse probability of treatment weights to include in a weighted Cox regression model. The 95% confidence intervals (CI) were estimated using a robust variance estimator to account for dependency between observations induced by weighting. Distribution of baseline covariates was assessed using standardized mean differences.20 Standardized mean differences below the arbitrary limit of 0.1 are usually considered a marker of sufficient balance. A 2-sided P value ≤.05 was considered statistically significant. Missing data were in all cases found to be missing at random, and were handled using chained multiple imputation (n = 15 sets). Statistical analyses were performed in Stata 16.1 for Windows (StataCorp, College Station, TX).
Results
We identified 41,039 consecutive first-time CIED patients. We excluded 334 patients who had epicardial or subcutaneous lead(s). In 79 patients we were unable to obtain generator data (n = 39), or linkage to DNPR was not possible (n = 40). Twenty-five patients were excluded owing to missing information about sex (n = 12) or unknown status in the Central Patient Registry (n = 13). The final cohort thus comprised 40,601 patients, including 2952 (7%) patients treated with TP. An ipsilateral permanent CIED implantation was performed in 164 (6%) TP patients (Supplemental Table 2). Median duration of TP was 6 days (interquartile range 3–11 days). Baseline characteristics according to TP status are presented in Table 1. Notably, TP patients were older and tended to have more risk factors for infection. After weighting, baseline characteristics were balanced for all covariates (Supplemental Table 2). Missing data were imputed for body mass index (n = 5452), C-reactive protein (n = 3155), and body temperature at implant (n = 3152).
Table 1.
Baseline characteristics in temporary transvenous pacing patients and non–temporary transvenous pacing patients in weighted and unweighted cohorts
| Total cohort (n=40,601) | Non-TP patients, n (%) | TP patients, n (%) | CIED infection, n (%) | IE hospitalization, n (%) |
|---|---|---|---|---|
| Total | 37,649 (100) | 2952 (100) | 246 (100) | 151 (100) |
| Age | ||||
| <60 years | 5391 (14.3) | 41 (20.3) | 26 (17.3) | 298 (10.1) |
| 60–69 years | 7857 (20.9) | 44 (21.8) | 27 (18.0) | 505 (17.1) |
| 70–79 years | 12,452 (33.1) | 65 (32.2) | 52 (34.7) | 996 (33.7) |
| ≥80 years | 11,949 (31.7) | 52 (25.7) | 45 (30.0) | 1153 (39.1) |
| Men | 23,985 (63.7) | 1,797 (60.9) | 185 (75.2) | 108 (72.0) |
| BMI† | ||||
| Low (<18.5) | 14,567 (40.8) | 109 (44.3) | 71 (47.3) | 1311 (47.3) |
| Normal (18.5–24.9) | 12,781 (35.8) | 89 (36.2) | 48 (32.0) | 1011 (36.5) |
| High (>25) | 5042 (14.1) | 48 (19.5) | 31 (20.7) | 437 (15.8) |
| University center | 19,994 (53.1) | 1903 (64.4) | 147 (59.8) | 93 (62) |
| Device type | ||||
| Pacemaker | 26,778 (71.1) | 133 (54.1) | 98 (65.3) | 2502 (84.8) |
| ICD | 6951 (18.5) | 70 (28.5) | 27 (18.0) | 132 (4.5) |
| CRT-P | 1623 (4.3) | 13 (5.3) | 13 (8.7) | 142 (4.8) |
| CRT-D | 2297 (6.1) | 30 (12.2) | 12 (8.0) | 176 (6) |
| Venous access site for CIED | ||||
| Cephalic | 23,469 (62.3) | 1786 (60.5) | 149 (60.6) | 79 (52.3) |
| Subclavian | 12,269 (32.6) | 949 (32.1) | 87 (35.4) | 61 (40.4) |
| Cephalic and subclavian | 1388 (3.7) | 189 (6.4) | 7 (2.8) | 11 (7.3) |
| Femoral/jugular/axillary | 20 (0.1) | 1 (0) | 1 (0) | 0 |
| Unknown | 503 (1.3) | 27 (0.9) | 2 (0.1) | 0 |
| Venous access site for TP | - | - | - | |
| Subclavian | - | 2047 (69.3) | - | - |
| Femoral | - | 230 (7.8) | - | - |
| Jugular/other | - | 504 (17.1) | - | - |
| Unknown | - | 159 (5.4) | - | - |
| Temperature ≥38°C† | 148 (0.4) | 21 (0.7) | 0 | 0 |
| C-reactive protein >8 mg/L† | 10,467 (30.3) | 1979 (67.0) | 91 (37) | 77 (51.3) |
| Chronic renal insufficiency | 2895 (7.7) | 366 (12.4) | 20 (8.1) | 19 (12.7) |
| Dialysis | 197 (0.5) | 3 (1.2) | 3 (2.0) | 49 (1.7) |
| Central venous catheter | 1566 (4.2) | 408 (13.8) | 25 (10.1) | 13 (8.6) |
| Diabetes mellitus | 6273 (16.7) | 599 (20.3) | 59 (24) | 36 (24.0) |
| Congestive heart failure | 12,076 (32.1) | 891 (30.2) | 109 (44.3) | 63 (42.0) |
| Ischemic heart disease | 17,092 (45.4) | 1384 (46.9) | 137 (55.7) | 82 (54.7) |
| Prior endocarditis diagnosis | 238 (0.6) | 124 (4.2) | 14 (6.7) | 0 |
| Prior valve replacement surgery | 1780 (4,7) | 566 (19.2) | 29 (11.8) | 42 (28.0) |
| Malignancy | 5626 (14.9) | 536 (18.2) | 27 (11) | 21 (14.0) |
| Connective tissue disease including rheumatoid arthritis | 1952 (5.2) | 237 (8.0) | 16 (6.5) | 8 (5.3) |
| Anticoagulant/antiplatelet therapy | 24,123 (64.1) | 1798 (60.9) | 172 (69.9) | 119 (79.3) |
| Vitamin K antagonists | 6917 (18.4) | 485 (16.4) | 50 (20.3) | 30 (20.0) |
| Immunosuppressant therapy | 2578 (6.9) | 254 (8.6) | 19 (7.7) | 14 (9.3) |
BMI = body mass index; CABG = coronary artery bypass graft; CRT-P/-D = cardiac resynchronization therapy pacemaker/-defibrillator; ICD = implantable cardioverter-defibrillator; TP = temporary transvenous pacing.
Missing values for temperature ≥38°C (n = 3152), C-reactive protein (n = 3155), and BMI group (n = 5452), handled using multiple imputation.
Cardiac implantable electronic device infections
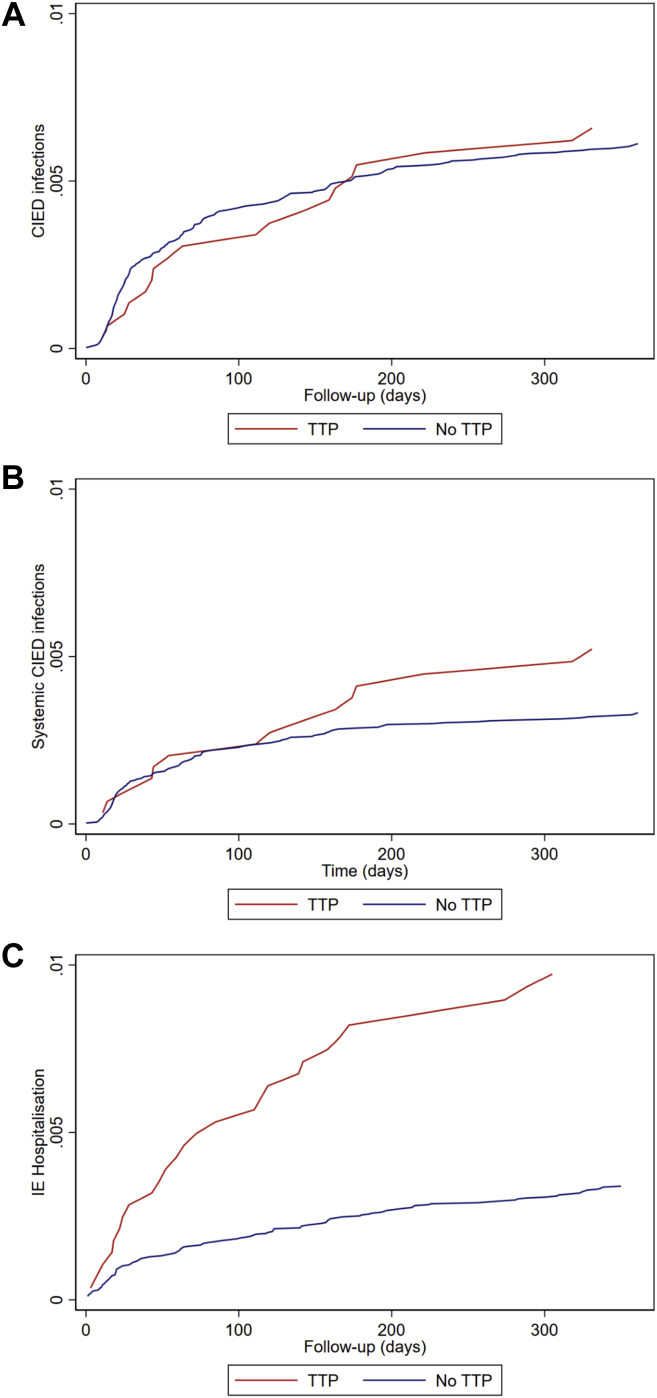
A total of 246 patients reached the primary outcome of CIED infection: 227 non-TP patients and 19 TP patients (Tables 1 and 2). One-year infection risks were 0.61% and 0.65% (Figure 1a), and event rates were 0.66 and 0.75 per 100 person-years (PY). The rate of CIED infection was not significantly different between groups: the unadjusted 1-year HR for CIED infection was 1.28 (95% CI 0.80–2.05), and 0.85 (95% CI 0.51–1.42) after adjustment (Table 2) for TP patients. Baseline characteristics for patients with CIED infections are listed in Table 1.
Table 2.
Infection risks and rates in temporary transvenous pacing patients and non–temporary transvenous pacing patients
| Events | Event rate per 100 PY | 1-year CIP (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Weighted HR† (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Any CIED infection | Non-TP | 227 | 0.66 (0.58–0.75) | 0.61 (0.53–0.69) | Ref. | Ref. | Ref. |
| TP | 19 | 0.75 (0.48–1.18) | 0.65 (0.36–0.95) | 1.28 (0.80–2.05) | 0.85 (0.51–1.42) | 0.56 (0.29–1.13) | |
| Systemic CIED infection | Non-TP | 123 | 0.36 (0.30–0.43) | 0.33 (0.27–0.39) | Ref. | Ref. | Ref. |
| TP | 15 | 0.60 (0.36–0.99) | 0.52 (0.26–0.79) | 1.84 (1.07–3.16) | 0.99 (0.54–1.80) | 0.74 (0.33–1.69) | |
| Hospitalization for IE‡ | Non-TP | 124 | 0.36 (0.31–0.43) | 0.33 (0.28–0.40) | Ref. | Ref. | Ref. |
| TP | 27 | 1.12 (0.7-1.63) | 0.97 (0.61-1.34) | 3.07 (2.01-4.68) | 1.68 (1.06-2.67) | 1.49 (0.89-2.51) | |
| All-cause mortality | Non-TP | 3158 | 8.82 (8.52–9.13) | 8.39 (8.11–8.67) | Ref. | Ref. | Ref. |
| TP | 497 | 18.87 (17.28–20.61) | 16.84 (15.53–18.23) | 1.92 (1.75–2.11) | 1.36 (1.13–1.64) | 1.57 (1.34–1.84) |
CIED = cardiac implantable electronic device; CIP = cumulative incidence proportion; IE = infective endocarditis; PS = propensity score; PY = person-years; TP = temporary transvenous pacing.
Estimated using stabilized inverse probability of treatment weights based on propensity scores.
Excluding patients with prior hospitalization(s) for endocarditis (n = 362).
Figure 1.
One-year unadjusted cumulative incidence curves for non–temporary transvenous pacing (TTP) patients and temporary transvenous pacing patients. a: Any cardiac implantable electronic device (CIED) infection resulting in device system removal. b: Systemic CIED infections resulting in device system removal. c: Hospitalization for infective endocarditis (IE).
For the secondary outcome of systemic CIED infection, we disregarded all cases of localized (pocket) CIED infections; 138 systemic CIED infections remained. Event rates per 100 PY in non-TP and TP patients were 0.36 and 0.60, and the 1-year risk of systemic CIED infections was 0.33% and 0.52%, respectively (Table 2, Figure 1b). The unadjusted HR was 1.84 (95% CI 1.07–3.16); however, confounder adjustment yielded a nonsignificant adjusted HR of 0.99 (95% CI 0.54–1.80) (Table 2) (results for both outcomes stratified by device type are listed in Supplemental Table 3 and visualized for PMs in Supplemental Figure 1).
Hospitalization for infective endocarditis
For the secondary outcome of IE hospitalization, we considered patients with ≥14-day admission for IE regardless of device system removal. As IE may be an indication for TP, we excluded 362 patients with hospitalizations for IE prior to the first CIED implantation to avoid misclassification owing to overflow of preimplant IE diagnoses to subsequent hospitalizations. This maneuver was necessary because both covariates (prior and subsequent IE) were drawn from DNPR, and we could not, with confidence, distinguish between new infections and diagnoses that may have been carried forward from previous admissions. The final cohort thus comprised 37,411 non-TP patients and 2828 TP patients (total cohort of 40,239 patients). In total, 151 patients were hospitalized for IE within the first year of implantation (Table 2). The event rates were 0.36 per 100 PY in non-TP patients and 1.12 per 100 PY in TP patients. Eleven of 151 patients died during an IE hospitalization (3 TP patients, 8 non-TP patients). The 1-year risk of hospitalization for IE was 0.34% and 0.97% in non-TP and TP patients, respectively (Figure 1c). We computed an unadjusted HR of 3.04 (95% CI 2.01–4.62). This relative increase in infection rate remained—although reduced—statistically significant after confounder adjustment, HR 1.68 (95% CI 1.06–2.67). However, statistical significance was lost in the weighted analysis (HR 1.49, 95% CI 0.89–2.51) (see Supplemental Table 3 for results stratified by device type).
Among TP patients, we observed that duration of TP was longer for patients hospitalized for IE (median duration 13 vs 6 days), and for patients who developed a CIED infection (median duration 8 vs 6 days) than for noninfected patients (Table 1). Moreover, prior valve replacement surgery was more prevalent among patients hospitalized for IE (28%) and particularly among infected TP patients.
All-cause mortality
A total of 3655 patients died during follow-up; 497 were TP patients. The 1-year risk of all-cause death for non-TP patients was 8.4% and for TP patients 16.8%. The all-cause mortality rate in TP patients was higher than in non-TP patients in crude and adjusted analyses, with HRs of 1.92 (95% CI 1.75–4.68) and 1.36 (95% CI 1.13–1.64) (Table 2).
Discussion
We conducted a nationwide cohort study to investigate the impact of TP on risk of subsequent CIED infection and IE. In a complete and contemporary cohort of first-time CIED patients, we found no association between TP and incidence of CIED infections—in neither crude nor adjusted analyses. Under the presumption that TP would predominantly increase risk of systemic infections, we repeated our analysis with exclusion of localized (pocket) infections; TP was associated with a higher rate of systemic CIED infections, but the association did not retain statistical significance after confounder adjustment. Conversely, the rate of IE hospitalizations was higher with TP even with adjustment, although statistical significance was lost in the weighted analysis. Overall infection rates were low and in concordance with previously reported rates for first CIED implantations.2 Irrespective of group, 1-year infection risks did not exceed 1% for any infection type.
Patients treated with TP were generally older at time of first CIED implantation, had more risk factors for CIED infection, and displayed overall worse outcomes. Indications for treatment with TP include ongoing infection and hemodynamic instability, which lead to selection of high-risk CIED patients. In effect, TP patients are almost definitional patients at risk. The 1-year cumulative mortality in TP patients was doubled compared to non-TP patients. Higher mortality rates in conjunction with a more pronounced burden of comorbidity in TP patients was recently described in 2 large observational cohort studies using data from the US National Inpatient Sample4 and the Australian Admission Patient Data Collection registry.6 Based on 4838 Australian TP patients,6 survival for patients treated with TP—including TP followed by permanent PM implantation—was worse than for patients treated with permanent PM alone even after adjustment for age, sex, and comorbidities. Data regarding CIED-related infections were not available for this study, but sepsis was a major cause of both in-hospital and postdischarge death among TP patients.
Results from this study regarding CIED infections contrast previous findings. A meta-analysis by Polyzos and colleagues10 reported a higher associated risk of CIED infections with TP based on results from 10 studies (odds ratio [OR] 2.31, 95% CI 1.36–3.92). However, common to all studies included was that none examined TP as their primary exposure of interest, a majority of the original studies were restricted to univariable or descriptive statistics based on few TP cases, and most were of older date, with publication dates ranging from 1995 to 2012 and cohorts dating even further back. The largest and most comprehensive study included was a prospective, multicenter registry study by Klug and colleagues,9 which included 6319 CIED patients and counted 42 infections at 12 months of follow-up. Use of TP was positively associated with CIED infections in both univariable (OR 3.58, 95% CI 1.48–8.65) and multivariable analyses (adjusted OR 2.46, 95% CI 1.09–8.27), although in both cases conferring wide CIs. One distinction between these 2 studies was our restriction to first-time implantations, which effectively eliminated significant risk factors such as multiple previous CIED procedures, early reintervention, and prior CIED infections. Finally, no adjustment for patient-related risk factors was made in the multivariable analysis reported by Klug and colleagues, which may have impacted results, given the accumulation of risk factors among TP patients observed in this and other studies.
In our study, we observed higher rates of IE hospitalizations in TP patients compared to non-TP patients even after covariate adjustment, and although not statistically significant in the weighted analysis, some signal was retained. This apparent inconsistency in results for systemic CIED infections (with CIED removal) and IE hospitalization (regardless of CIED removal) is surprising, as a definite diagnosis of IE would today prompt device system removal. There are several possible explanations. First, infected patients who die and patients either too frail or unwilling to undergo extraction are not registered, as a registration of infection presupposes system extraction. However, abandonment of extraction owing to frailty is less likely in our patients, as follow-up was restricted to 1 year. Secondly, CIED infections remain a challenging diagnosis, as clinical evidence of CIED involvement may be absent.21 The evidence base for management of occult septicemia with pathogens associated with CIED infections is sparse, and choice of treatment may have relied on institutional guidelines or physician preference. With diagnostic uncertainty, physicians may have opted for conservative treatment strategies. Finally, a discharge diagnosis of IE does not necessarily reflect clinical reality. A Danish study that aimed to investigate risk of IE and CIEDs in patients undergoing aortic valve replacement surgery likewise suggested that low extraction rates observed in IE CIED patients (only 35%) could have been due to short life expectancy or absent clinical suspicion of infected right-sided leads with left-sided IE.22 A high proportion of our IE CIED patients had a history of valve replacement surgery, and this was particularly pronounced among TP patients (Supplemental Table 4).
Strengths and limitations
A limitation of this study is—as is implied in all observational studies—unknown or unmeasured confounding. Although efforts were made to control for confounding using both conventional multivariable regression and propensity scores, residual confounding cannot be dismissed. TP in pacing-dependent patients often represents a last-resort treatment (eg, in cases of infection or in the critically ill). Frailty in these patients is difficult to quantify and may have confounded our results. Even with adjustment, TP appeared to be independently associated with a higher mortality, which could be suggestive of residual confounding. With the exception of younger age, factors that tend to increase risk of CIED infection or IE are also generally associated with higher mortality. Consequent to this, we find it unlikely that any such unmeasured confounder would influence our main results to an extent that would lead to opposite conclusions.
Unfortunately, information about indications for TP was not available through our registries. In Denmark, CIED implantations are centralized to few centers, and all are able to perform implantations within 24 hours on any given day. In essence, TP is only applied in cases of ongoing infection, in acute need of pacing outside daytime in some centers, or in settings of an uncertain clinical outcome or need of pacing while awaiting a more complex CIED system such as an ICD or CRT. In addition, prescription registries do not offer information about medication provided by hospitals, including chemotherapeutics and certain immunosuppressant drugs used for autoimmune diseases. However, adjustment was made for both malignancy and connective tissue disorders.
Proper classification of clinical outcomes using retrospective data remains a challenge. While we find it unlikely that patients who undergo device system removal owing to infection are not recorded, registration assumes system extraction and when circumstances impede so, patients are misclassified. Moreover, clinical registries reflect clinical practice and changes in—or implementation of—guidelines over time influence who is registered and who is not. Taken together, we may have underestimated the true number of CIED infections using DPIR.
Finally, we included patients at time of first implantation, as our objective was to investigate infections in relation to the permanent CIED. As such, TP patients with transient pacing requirements were not included, and an assessment of complications specifically relating to the temporary pacing wire was beyond the scope of this study.
Despite limitations implicit to observational studies, use of registry data is invaluable for studies of rare exposures and outcomes, as is the case with CIED infections after TP. CIED technologies continue to evolve, as does the pool of possible recipients. The rise in implant activity worldwide has triggered a parallel—perhaps even accelerated—rise in CIED infections. To counter this trend, we need accurate knowledge about the safety and risks associated with CIED therapy in contemporary populations, to guide future practice, target prevention, and ensure proper information to patients. Although we found no association between TP and infection in the fully adjusted models, these patients do appear to be more susceptible to systemic infections with cardiac involvement, and this should warrant special attention to this patient group.
Conclusion
In a nationwide, Danish cohort study including more than 40,000 consecutive first-time CIED patients, active-fixation TP was not associated with a higher rate of all-cause CIED infections. The rate of systemic CIED infections and IE hospitalizations (with or without CIED removal) was higher with TP. This could be attributed to an accumulation of risk factors for infection among patients treated with TP. This predisposition for infection among TP patients likely arises from selection of high-risk individuals through treatment indication. Although TP did not appear to independently increase risk of infection, our study reinforced the fact that patients selected for TP are at high risk of systemic infections, and any permanent CIED implantation in patients previously treated with TP should urge special care and considerations.
Acknowledgments
Funding Sources
This work was supported by a grant from the Karen Elise Jensen’s Foundation.
Disclosures
J.C.N. is supported by grants from the Novo Nordisk Foundation [NNF16OC0018658, NNF17OC0029148]. J.B.J. reports personal fees from Medtronic, personal fees from Biotronik, and nonfinancial support from Merit Medical, outside the submitted work. Remaining authors declare no conflicts of interest.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Register-based studies do not require informed consent from patients, according to Danish law.
Ethics Statement
This study was approved by the Central Denmark Region (1-16-02-199-18) and the DPIR Steering Committee. According to Danish law, register-based studies do not require ethics committee approval.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2021.11.008.
Appendix. Supplementary data
References
- 1.Glikson M., Nielsen J.C., Kronborg M.B., et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) with the special contribution of the European Heart Rhythm Association (EHRA) Europace. 2021 doi: 10.1093/europace/euab232. Accessed December 18, 2021. [DOI] [Google Scholar]
- 2.Blomstrom-Lundqvist C., Traykov V., Erba P.A., et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) Eur J Cardiothorac Surg. 2020;57:e1–e31. doi: 10.1093/ejcts/ezz296. [DOI] [PubMed] [Google Scholar]
- 3.Kusumoto F.M., Schoenfeld M.H., Wilkoff B.L., et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14:e503–e551. doi: 10.1016/j.hrthm.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Metkus T.S., Schulman S.P., Marine J.E., Eid S.M. Complications and outcomes of temporary transvenous pacing: an analysis of > 360,000 patients from the national inpatient sample. Chest. 2019;155:749–757. doi: 10.1016/j.chest.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 5.de Cock C.C., Van Campen C.M., In't Veld J.A., Visser C.A. Utility and safety of prolonged temporary transvenous pacing using an active-fixation lead: comparison with a conventional lead. Pacing Clin Electrophysiol. 2003;26:1245–1248. doi: 10.1046/j.1460-9592.2003.t01-1-00175.x. [DOI] [PubMed] [Google Scholar]
- 6.Ng A.C.C., Lau J.K., Chow V., et al. Outcomes of 4838 patients requiring temporary transvenous cardiac pacing: a statewide cohort study. Int J Cardiol. 2018;271:98–104. doi: 10.1016/j.ijcard.2018.05.112. [DOI] [PubMed] [Google Scholar]
- 7.García Guerrero J.J., Fernández de la Concha Castañeda J., López Quero D., et al. Lower incidence of venous thrombosis with temporary active-fixation lead implantation in mobile patients. Europace. 2010;12:1604–1607. doi: 10.1093/europace/euq262. [DOI] [PubMed] [Google Scholar]
- 8.Sanders P., Farouque H.M., Ashby D.T., Mahar L.J., Young G.D. Effect of anticoagulation on the occurrence of deep venous thrombosis associated with temporary transvenous femoral pacemakers. Am J Cardiol. 2001;88:798–801. doi: 10.1016/s0002-9149(01)01857-4. [DOI] [PubMed] [Google Scholar]
- 9.Klug D., Balde M., Pavin D., et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007;116:1349–1355. doi: 10.1161/CIRCULATIONAHA.106.678664. [DOI] [PubMed] [Google Scholar]
- 10.Polyzos K.A., Konstantelias A.A., Falagas M.E. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace. 2015;17:767–777. doi: 10.1093/europace/euv053. [DOI] [PubMed] [Google Scholar]
- 11.de Cock C.C., Van Campen L.C., Visser C.A. Usefulness of a new active-fixation lead in transvenous temporary pacing from the femoral approach. Pacing Clin Electrophysiol. 2003;26:849–852. doi: 10.1046/j.1460-9592.2003.t01-1-00149.x. [DOI] [PubMed] [Google Scholar]
- 12.Kawata H., Pretorius V., Phan H., et al. Utility and safety of temporary pacing using active fixation leads and externalized re-usable permanent pacemakers after lead extraction. Europace. 2013;15:1287–1291. doi: 10.1093/europace/eut045. [DOI] [PubMed] [Google Scholar]
- 13.Braun M.U., Rauwolf T., Bock M., et al. Percutaneous lead implantation connected to an external device in stimulation-dependent patients with systemic infection--a prospective and controlled study. Pacing Clin Electrophysiol. 2006;29:875–879. doi: 10.1111/j.1540-8159.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 14.Cipriano R., Gupta A., Subzposh F., et al. Outcomes of standard permanent active fixation leads for temporary pacing. JACC Clin Electrophysiol. 2020;6:304–310. doi: 10.1016/j.jacep.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Pottegård A., Schmidt S.A.J., Wallach-Kildemoes H., et al. Data resource profile: the Danish National Prescription Registry. Int J Epidemiol. 2016;46 doi: 10.1093/ije/dyw213. 798–798f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundbøll J., Adelborg K., Munch T., et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adelborg K., Sundbøll J., Munch T., et al. Positive predictive value of cardiac examination, procedure and surgery codes in the Danish National Patient Registry: a population-based validation study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-012817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Østergaard L., Adelborg K., Sundbøll J., et al. Positive predictive value of infective endocarditis in the Danish National Patient Registry: a validation study. Epidemiol Infect. 2018;146:1965–1967. doi: 10.1017/S0950268818001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brookhart M.A., Schneeweiss S., Rothman K.J., et al. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin P.C., Stuart E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakajima I., Narui R., Tokutake K., et al. Staphylococcus bacteremia without evidence of a cardiac implantable electronic device infection. Heart Rhythm. 2021;18:752–759. doi: 10.1016/j.hrthm.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Ostergaard L., Valeur N., Bundgaard H., et al. Cardiac implantable electronic device and associated risk of infective endocarditis in patients undergoing aortic valve replacement. Europace. 2018;20:e164–e170. doi: 10.1093/europace/eux360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.