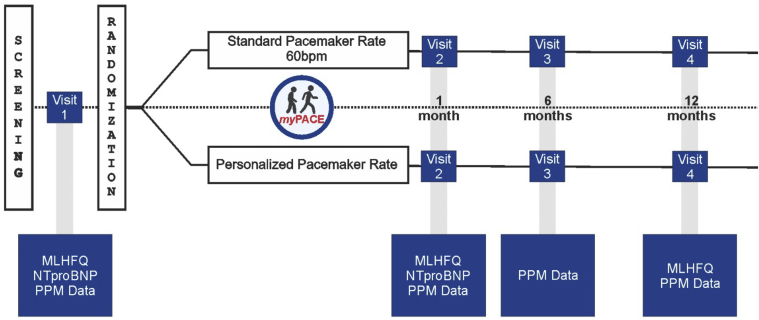
Figure 3.
myPACE study flowchart. Patients scheduled in our pacemaker clinic are consecutively screened. Those patients who are enrolled complete a baseline MLHFQ quality-of-life score, NTproBNP, and a pacemaker interrogation. Patients are randomized to either the myPACE or the control group. NTproBNP was repeated at 1 month, and MLHFQ was repeated at 1 month and 1 year. Pacemaker-detected data and clinical outcomes are monitored during the 1-year study period. MLHFQ = Minnesota Living with Heart Failure Questionnaire; NTproBNP = N-terminal pro–brain natriuretic peptide; PPM = permanent pacemaker.