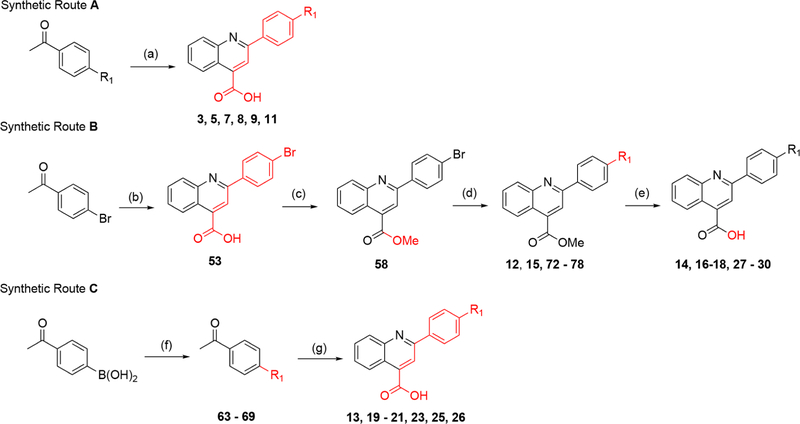
Scheme 1. Synthesis of Quinoline Analogues without a C3 Methyl Groupa.
aReagents and conditions. Route A: (a) isatin, KOH, EtOH/H2O reflux, 12–48 h (10–41%). Route B: (b) isatin, KOH, EtOH/H2O, 12–48 h (81%); (c) MeI, Cs2CO3 DMF, rt, overnight (43%); (d) R1-boronic acid/ester, Pd(PPh3)4, K2HPO4, dioxane/H2O, 130 °C, 1.5 h (7–93%); (e) LiOH or NaOH, THF/H2O, rt, overnight (15–100%). Route C: (f) R1-halogen, Pd(PPh3)4, K2HPO4, dioxane/H2O, 130 °C, 1.5 h (13–97%); (g) isatin, KOH, EtOH/H2O, reflux; 12–48 h (18–57%).