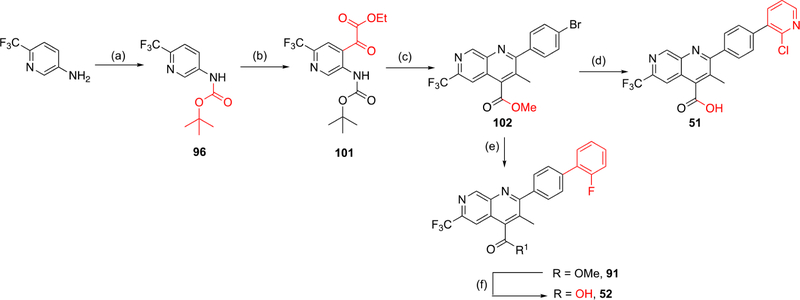
Scheme 4. Alternate Synthesis of Naphthyridine Core Analoguesa.
aReagents and conditions. Route F: (a) Boc2O, p-dioxane, reflux 72 h (36%); (b) (1) n-BuLi, TMEDA, Et2O, −78 to 10 °C (15 min to 2.5 h), (2) diethyl oxalate, Et2O, −78 °C to rt, 1.5 h (l%); (c) (1) TFA, DCM, rt, 2 h, (2) 4′-bromopropiophenone, KOH, EtOH, 100 °C, 24 h, (3) TMS-diazomethane, THF, MeOH, rt, 2 h (2% over three steps), (d) (1) NaOH, dioxane, 60 °C, overnight, (2) 2-chloropyridine-3-boronic acid, Pd(PPh3)4, K2HPO4, dioxane/H2O, 130 °C, 1.5 h (46% over two steps); (e) 2-fluorophenylboronic acid, Pd(PPh3)4, K2HPO4 dioxane/H2O, 130 °C, 1.5 h (66%); (f) KOH, THF/H2O, 50 °C, 4 h (50%).