Figure 3. Loss of FTO increases DKK1 expression.
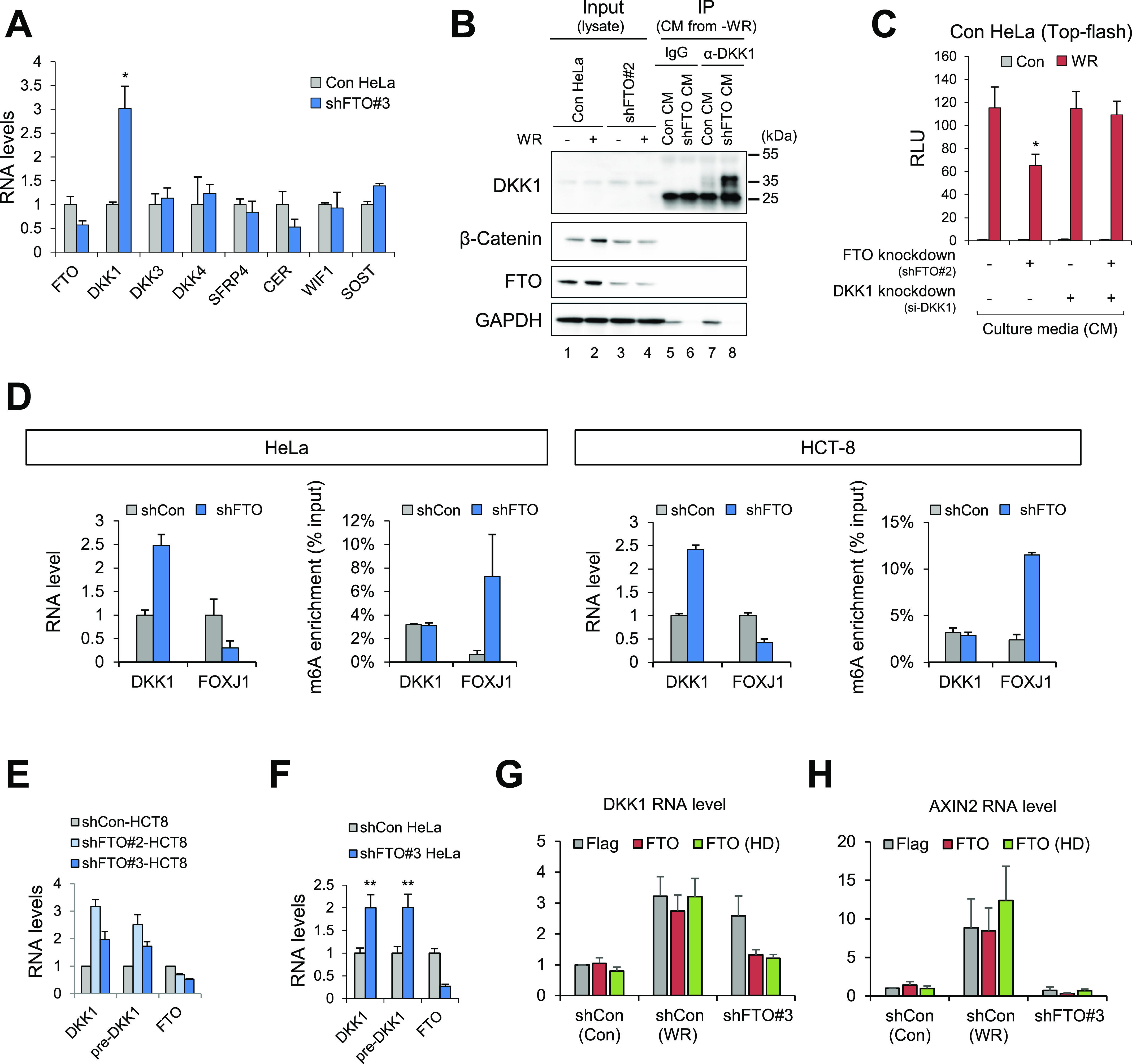
(A) RNA levels of various extracellular WNT antagonists were measured by RT-qPCR from control or FTO-depleted HeLa cells (shFTO#3). GAPDH was used as a normalization control (n = 3). (B) Secreted DKK1 protein levels were measured by Western blot after immunoprecipitating DKK1 from culture medium of control (Con CM) or FTO-depleted (shFTO#2) HeLa cells (shFTO CM). IgG immunoprecipitation serves as a negative control. (C) Culture medium of HeLa cells transiently expressing WNT reporter (TOP-flash) was replaced with the medium harvested from DKK1 siRNA or control siRNA (si-NC) transfected HeLa cells (control or FTO-depleted). Relative luciferase activities were measured after 16 h of WNT stimulation (WR, WNT3a, and R-Spondin1) (n = 6). (D) Unfragmented poly(A)+ RNAs from control or FTO-depleted HeLa or HCT-8 cells were immunoprecipitated with anti-m6A antibody. m6A enrichments were calculated as relative amounts of m6A immunoprecipitated fraction compared with input. Fold changes were compared after GAPDH normalization between input samples of control and FTO depletion (n = 3). (E) RNA levels of mature and pre-spliced form of DKK1 (pre-DKK1) were measured by RT-qPCR from shCon or two different shFTO (#2 and #3) expressing HCT-8 cells. GAPDH was used as a normalization control (n = 3). (F) RNA levels of mature and pre-spliced form of DKK1 were measured by RT-qPCR from control or FTO-depleted HeLa cells (shFTO#3). GAPDH was used as a normalization control (n = 3). (G) RNA levels of DKK1 were measured by RT-qPCR from control or FTO-depleted cells transfected with empty Flag vector, wild-type FTO or catalytically inactive FTO (FTO HD) treated with control or WR (n = 3). (H) RNA levels of AXIN2 were measured by RT-qPCR from control or FTO-depleted cells transfected with empty Flag vector, wild-type FTO or catalytically inactive FTO (FTO HD) treated with control or WR (n = 3). GAPDH was used as a loading control for Western blot. WR, WNT3A, and R-Spondin1. Error bars are ±SEM. One-sided t test, *P < 0.05, **P < 0.01.
