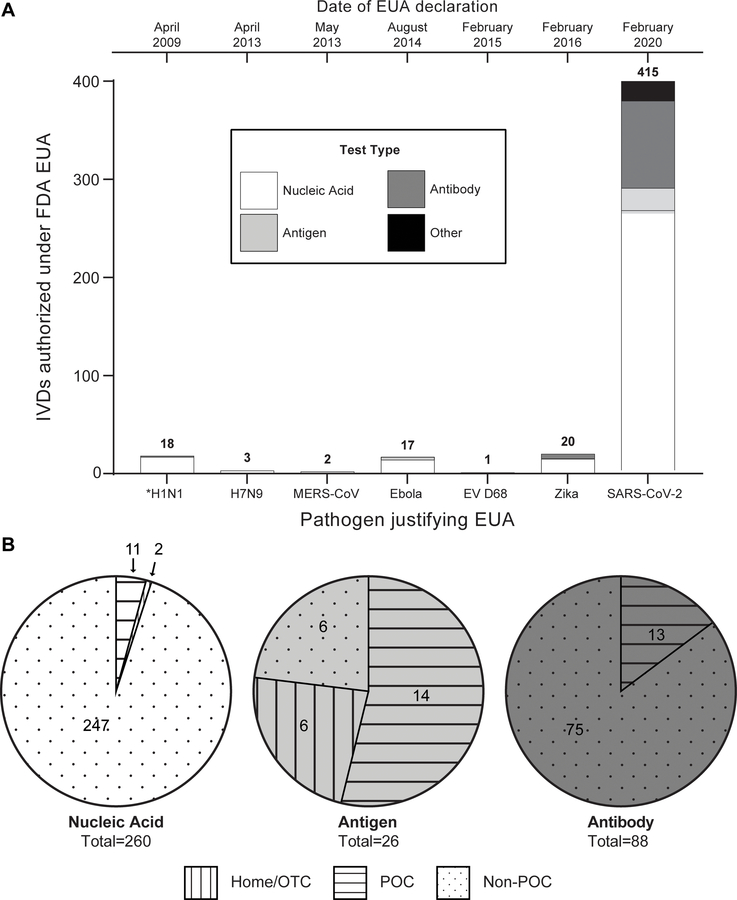
Figure 4. Overview of IVD’s that have received FDA EUA.
A) Total number of IVD’s issued EUA by the FDA during previous declarations of the EUA pathway. Note: IVD’s that have had their EUA revoked are included in the analysis, and all listed pathogens here have current EUAs except H1N1 (designated with *). B) Breakdown of current SARS-CoV-2 IVDs that have received EUA by assay type (nucleic acid, antigen, antibody) and authorized setting to run the test. See ESI for more information on methods for data compilation.