Table 3.
Antiviral effect of FA derivatives.
| Derivative | General structure | Virus | Antiviral activity/Cytotoxicity of the compound | Structure | References |
|---|---|---|---|---|---|
| Chemically synthesized | |||||
| FA polymers |
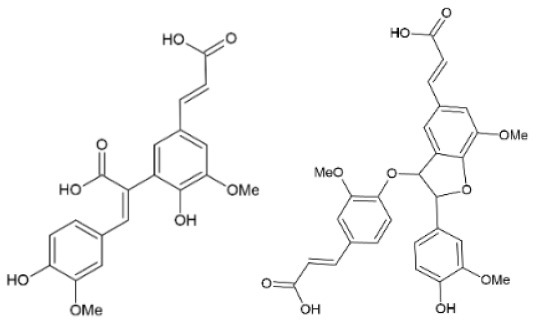
|
HIV-1 | IC50 = 1.0 μg/ml IC50 = 0.9 μg/ml IC50 = 2.7 μg/ml IC50 = 3.2 μg/ml |
>30 kDa 30–10 kDa 10–1 kDa 1 kDa−500 Da |
(104) |
| Example: diferulate, triferulate | |||||
| FA amide of 3-aminomethyl glaucine |
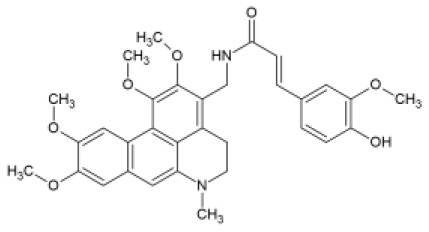
|
HRV-14 | IC50 = 12.00 μM CC50= 113 μM |
- | (105) |
| FA amides |
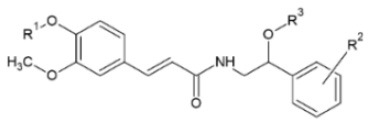
|
TMV | 40.7% protective effect 46.4% curative effect |
R1: n-Pr; R2: H R3: propynyl |
(106) |
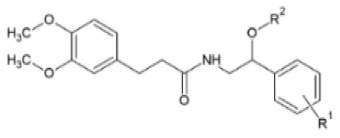
| Hydrogenated FA amides |
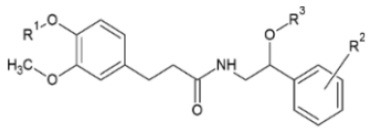
|
TMV | 27.3% protective effect 30.4% curative effect |
R1: Me R2: p-Cl R3: propynyl |
(106) |
|
TMV | 15.2% protective effect 28.9% curative effect |
R1: p-benzyloxy R2: propynyl |
(107) | |
| α,β-unsaturated amide derivatives of FA with an α-aminophosphonate moiety |
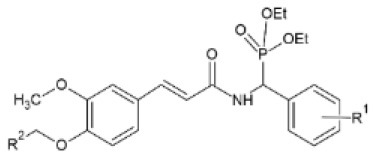
|
TMV | EC50 = 180.37 μg/ml (protective effect) | R1: 4-Cl | (108) |
| EC50 = 285.42 μg/ml (curative effect) | R2: 4-CF3-Ph | ||||
| CMV | EC50 = 216.30 μg/ml (protective effect) | ||||
| EC50 = 284.67 μg/ml (curative effect) | |||||
| Trans-FA derivatives containing acylhydrazone moiety |
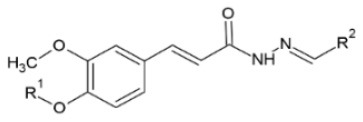
|
TMV | 18.8% curative effect | R1: PhCH2 | (109) |
| 23.0% protective effect | R2: 2-Th | ||||
| 94.2% inactivating effect | |||||
| EC50 = 36.59 μg/ml | |||||
| FA sulfonamides |
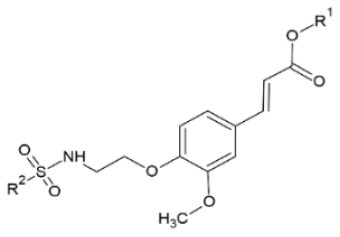
|
TMV | 39.8% curative effect | R1: -C2H5 | (110) |
| 59.7% protective effect 87.3% inactivating effect |
R2: 4-NO2-Ph | ||||
| EC50 = 84.80 μg/ml | |||||
| Myricetin derivatives with an FA amide scaffold |
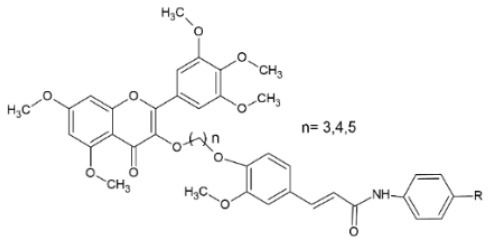
|
TMV | 55.5% curative effect 53.3% protective effect EC50 = 472.4 μg/ml |
R: 4-Br, n = 3 | (111) |
| FA 3-amino derivatives/esters |
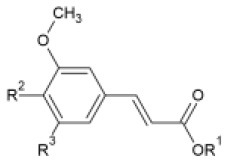
|
H1N1 | IC50 =90.0 μg/ml | R1: CH2(CH3) | (78) |
| R2: CH(CH3)2 | |||||
| R3: NO2 | |||||
| FA derivatives with a quinazoline moiety |
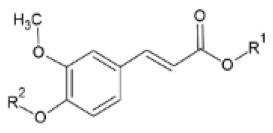
|
TMV | 60.8% curative effect | R1: 2-OCH3-Ph | (112) |
| 78.2% protective effect | R2: 4-oxoquinazolin-3(4H)-yl-methyl | ||||
| 90.8% inactivating effect | |||||
| CMV | 58.1% curative effect | R1: 2-OCH3-4-allyl-Ph | |||
| 69.8% protective effect | |||||
| R2:4-oxoquinazolin-3(4H)-yl-methyl | |||||
| 78.2% inactivating effect | |||||
| Trans-FA esters with a chalcone group |
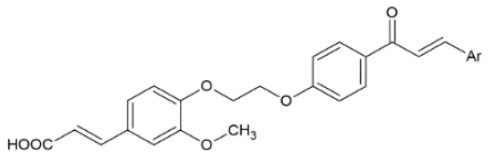
|
TMV | 63.9% curative effect | R: Me | (113) |
| 64.6% protective effect | Ar: 2-F-Ph | ||||
| 92.3% inactivating effect | |||||
| EC50 = 214.20 μg/ml | |||||
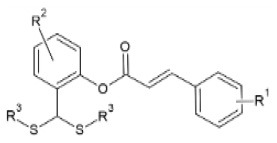
| FA derivatives containing dithioacetal moiety |

|
TMV | 62.7% curative effect | R1: 3-OCH3-4-OCOCH3 | (114) |
| 52.3% protective effect | |||||
| 73.8% inactivating effect | R2: 2-OCH3 | ||||
| EC50=73.7 μg/ml | R3: -(CH2)2OH | ||||
|
TMV | 62.5% curative effect | R1: 3-OCH3-4-OCOCH3 | ||
| 61.8% protective effect | R2: H | ||||
| R3: -(CH2)2OH | |||||
| 83.5% inactivating effect | |||||
| EC50= 50.7 μg/ml | |||||
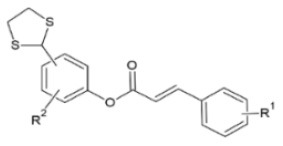
|
TMV | 48.% curative effect | R1: H | ||
| 48.% protective effect | R2:H | ||||
| 53.% inactivating effect | |||||
| EC50=355.6 μg/ml | |||||
| Enzymatically synthesized | |||||
| FA rutinoside |
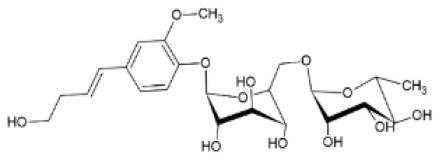
|
FCV | 40% increase of cell viability | – | (117) |
| FA β-sitosterol ester |
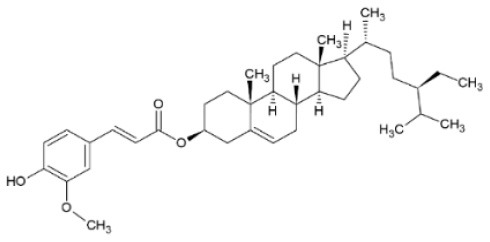
|
SARS-CoV-2 | Binding energy = −7.8 kcal/mol (molecular docking simulation with SARS-CoV-2 3CLpro) | – | (127) |
| (128) | |||||
HIV, human immunodeficiency virus; HRV, human rhinovirus; TMV, tobacco mosaic virus; CMV, cucumber mosaic virus; H1N1, influenza A virus subtype; FCV, feline calicivirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus; EC50, half-maximal effective concentration; the concentration required to obtain a 50% inhibitory effect; IC50, half-maximal inhibitory concentration; the concentration at which the number of viral plaques is reduced by 50%; CC50, half-maximal cytotoxicity concentration, the concentration that reduced cell viability to 50% of the control.
