ABSTRACT
Objectives:
Compared to formula-fed infants, breastfed infants have a lower risk of infections. Two possible reasons for this are the presence of the anti-infective and anti-inflammatory protein lactoferrin and the lower level of iron in breast milk. We explored how adding bovine lactoferrin and reducing the iron concentration in infant formula affect immunology and risk of infections in healthy infants.
Methods:
In a double-blind controlled trial, term formula-fed (FF) Swedish infants (n = 180) were randomized to receive, from 6 weeks to 6 months of age, a low-iron formula (2 mg/L) with added bovine lactoferrin (1.0 g/L) (Lf+; n = 72); low-iron formula with no added lactoferrin (Lf−; n = 72); and standard formula at 8 mg/L iron and no added lactoferrin (control formula [CF]; n = 36). Cytokines, infections, and infection related treatments were assessed until 12 months of age.
Results:
No adverse effects were observed. There were no apparent effects on transforming growth factor beta (TGF-β)1, TGF-β2, tumor necrosis factor alfa (TNF-α) or interleukin2 (IL-2) at 4, 6, or 12 months, except of higher TGF-β2 at 6 months in the CF group in comparison to the low iron groups combined (P = 0.033). No significant differences in otitis, respiratory infections, gastroenteritis, or other monitored infections and treatments were detected for any of the study feeding groups during the first 6 months and only a few and diverging effects were observed between 6 and 12 months.
Conclusions:
Adding bovine lactoferrin and reducing iron from 8 to 2 mg/L in infant formula was safe. No clinically relevant effects on cytokines or infection related morbidity were observed in this well-nourished and healthy population.
Keywords: cytokines, infant nutrition, infections
What Is Known/What Is New
What Is Known
Breastfeeding compared to formula feeding, is associated with reduced risk of infections and a different maturation of the inflammatory response.
Many infants worldwide are dependent on infant formula for nutrition.
Compared to human milk, standard infant formula has higher concentration of iron and lower level of lactoferrin.
What Is New
Lowering iron content and adding bovine lactoferrin in infant formula had no relevant effects on cytokines and was safe with regard to adverse events.
Lowering iron content and adding lactoferrin in infant formula had no effect on infection related morbidity in infants living in a context with a low burden of infectious diseases.
Breast milk is considered the optimal source of nutrition for infants and among several beneficial outcomes, studies have shown that breastfeeding is associated with reduced risk of diarrhea, respiratory infections and hospital admission due to these disorders, and also suggest a protective effect against otitis media, dental malocclusions and infant deaths as compared to infants fed formula (1). Still, many infants worldwide receive partial or complete feeding with infant formula, and for infants who cannot be breastfed, efforts to further improve the composition of infant formula may provide substantial benefits to infant health, particularly regarding immunological development and risk of infections.
Suggested mechanisms behind the reduced risk of infections include the effects of several bioactive proteins, promoting the immunological programming of the newborn infant (2,3). The abundant human milk protein lactoferrin has been envisioned as a particularly promising candidate. Lactoferrin supports the development of immature organs, the immune system and iron homeostasis (2,4,5). Clinical trials of bovine lactoferrin, as supplement or added to infant formula, have suggested a reduced risk of infections (6–9). The results are, however, diverging (10–13) and the effects on immunological markers, gut microflora and immune function are not known. Yet, bovine lactoferrin is not commonly added to infant formula.
Another striking difference between infant formula as compared to breast milk is the more than 20-fold higher concentration of iron (14,15). Reducing the risk of iron deficiency (ID) has been given high priority due to its association with impaired neurological development; however, iron excess may have toxic, pro-oxidative effects, and adverse effects such as increased risk of diarrhea and reduced growth, have been reported in infants (16–19). The optimal concentration of iron in infant formula, including the risk of infections and long-term effects on mental development, has not been fully evaluated in clinical trials (14).
The LIME project (Swedish acronym) aimed to investigate two interventions regarding infant formula composition. First, a reduction of iron concentration from 8 to 2 mg/L and second, an addition of 1 g/L of bovine lactoferrin. The effects on iron status and growth have been published previously (20). In this second paper from the study, we report the effects on immunological development including the cytokine profile (main outcome in the lactoferrin-intervention) and the prevalence of infections and other infection related morbidity and treatments (secondary outcomes for both interventions).
METHODS
Study Design and Participants
In a randomized, double-blind controlled infant formula trial, 180 exclusively formula-fed infants were included. The inclusion included equal sex distribution and was based on the following criteria: birth weight 2500–4500 g, gestational age at birth >37 weeks, absence of chronic illness and neonatal diagnoses likely to affect any outcome, and no previous blood transfusion or iron supplementation. Seventy-two breastfed infants were also registered as a reference group using the same inclusion criteria and an intention from parents to exclusively breastfeed until 6 months of age. Infants possible for recruitment were identified from delivery records at Umeå University hospital, Umeå, Sweden, or the nearby county hospital in Örnsköldsvik, and their parents were contacted by telephone. Only families who already had the intention to exclusively breastfeed or formula feed their infant respectively were considered as eligible for inclusion and given oral and written information. Parents who accepted participation gave written informed consent. The study was approved by the Regional Ethical Review Board in Umeå (ref 2013-245-31M) and registered at clinicaltrials.gov (NCT02103205).
Intervention
Using a computer-generated randomization tool, included formula-fed infants were stratified for sex and assigned randomly in blocks of 10, to receive a low iron (2 mg/L) formula fortified with bovine lactoferrin (1.0 g/L) (Lf+, n = 72), a low iron (2 mg/L) formula without lactoferrin fortification (Lf−, n = 72), or the same formula with a higher concentration of iron (8 mg/L) and no added lactoferrin (Control formula, CF, n = 36) (Fig. 1). To ensure blinding each of the low iron formula groups (Lf+ and Lf−) was represented by two assigned color groups (each of 36 infants). Both parents and staff were blinded to study group assignment until all infants had passed the follow-up at 1 year of age. The distribution of the formula was divided on several occasions during the intervention and coordinated by the study staff to assure compliance. Furthermore, the compliance to study feeding, and total daily iron intake, was monitored using a 3-day food diary collected for each month (20).
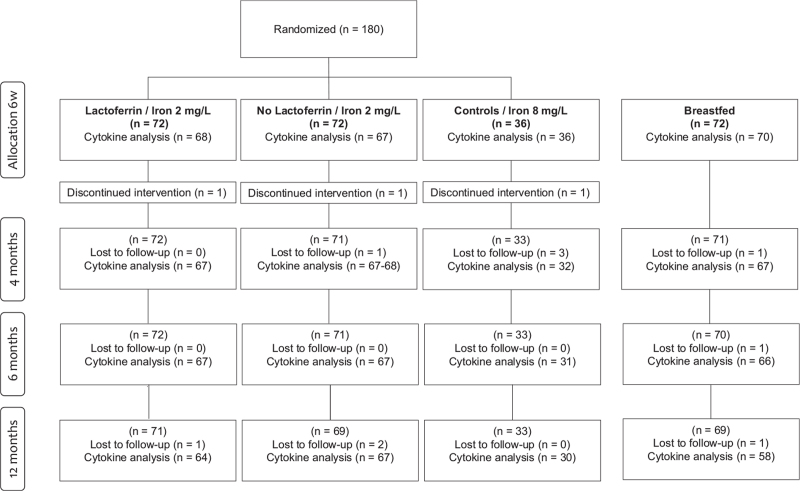
FIGURE 1.
Trial profile. Three infants discontinued intervention due to gastrointestinal symptoms (n = 2, CF, LF+) and extensive eczema (n = 1, LF−). They remained in the study. Six infants were lost to follow-up due to early withdrawal (n = 2, Lf−, BF), gastrointestinal side effects (n = 3, CF) and extensive data collection (n = 1, BF). Additional four infants were lost to follow up at 12 months of age (reasons not given). BF = breastfed; CF = control formula; Lf− = no added lactoferrin; Lf+ = added lactoferrin.
The control formula was a standard formula produced by Mead Johnson Nutrition, Evansville, IN, USA, with a lowered iron concentration (from 12 to 8 mg/L) to meet European standard infant formula recommendations. The Lf+ and Lf− formulas were further modified by reducing the iron concentration to 2 mg/L, and fortified (Lf+) with 1.0 g/L bovine lactoferrin (Hilmar Ingredients, Hilmar, CA, USA). Formula composition is summarized in Table 1, Supplemental Digital Content. In accordance with the World Health Organization (WHO) and national recommendations, the parents were asked to exclusively feed the assigned study feeding until at least 4 months of age and recommended to keep complementary foods to a minimum (taste portions) until 6 months of age. Products with added pre- or probiotics were not allowed.
Data Collection
Background information, including perinatal data, socio-economic data, and medical record of the infant, were collected from parents and delivery records at inclusion. The infants visited the study center at baseline (6 ± 2 weeks of age) and at 4, 6, and 12 months of age. The assessments included anthropometric data as reported elsewhere (20). Morbidity was monitored from inclusion to 12 months of age by a parent-registered daily diary of symptoms, medications, health care visits and hospitalization, together with a follow-up health-questionnaire at each visit. Verification from medical records was used if necessary. The following morbidity outcomes were monitored: fever (>38.0°C), cough, breathing difficulties, cold, otitis media, gastroenteritis, stomachache, sleeping disturbances, eczema or rash, medications, doctor visits, hospital stays and diagnosis of chronic disease. The parents received oral and written information how to fill in the diary of symptoms. Except for fever, it was left to each child's caregiver to determine whether symptoms were present or not and families were encouraged to contact the research nurses if there were any concerns. Data were analyzed as longitudinal prevalence (proportion of days with symptoms) or cumulative incidence (proportion of infants who present an outcome at least once). Symptom diaries lacking >30 registered days were excluded in longitudinal prevalence calculations.
Venous blood samples were drawn at each visit following a minimum of 2 hours fasting and 1 hour after application of local anesthetic topical cream (combination of Lidocaine 25 mg/g and Prilocaine 25 mg/g). Ethylenediaminetetraacetic acid (EDTA)-treated blood and a serum tube were sent to the local hospital laboratory for direct analyses of hemoglobin and iron status as a safety control of the interventions. A second tube with serum was centrifuged at 3000 rpm, divided into microtubes and stored frozen at −80oC until analyzed at the Pediatric Research lab at Umeå University for complementary iron status analyses as presented elsewhere (20), C-reactive protein (CRP) (R&D Systems Inc, Minneapolis, MN, USA), interleukin 2 (IL-2) and tumor necrosis factor alfa (TNF-α) (Milliplex Human High Sensitivity T Cell Magnetic Bead 2-plex Panel [HSTCMAG-28SK-02; EMD Millipore], Merck KGaA, Germany), and transforming growth factor beta (TGF-β) 1 and 2 (Milliplex TGF-β Magnetic Bead 3-plex Kit [TGFBMAG-64K-03; EMD Millipore], Merck KGaA, Germany). All analyses were performed in duplicate and samples with a coefficient of variation above 20%, or with values falling outside the measuring range were re-assayed at a different dilution. Cytokine measurements were carried out using a Bio-Plex 200 instrument (Bio-Rad Laboratories, Hercules, CA, USA). Concentrations of IL-2 and TNF-α were read from a 7-point calibration curve and TGF-β1 and -β2 from a 6-point calibration curve, and the calculations were made using Bio-Plex Manager 6.2 (Bio-Rad Laboratories, Hercules, CA, USA).
Sample Size and Statistical Analyses
In a pre-study power calculation, a minimum sample size of 64 in each randomization group was required to detect a difference of 0.5 standard deviation (SD), corresponding to a difference in geometric mean for IL-2 of 2.6 pg/mL and for TNF-α of 1.7 pg/mL, using a power of 80% and a significance level of 0.05 (21). The group size of the high iron group (CF) was set to a minimum of 24 based on the power calculation to detect a difference of 20 μg/L in geometric mean ferritin at 6 months compared to the combined low iron groups (n > 128). With an assumed attrition rate of 10% until 6 months, and to simplify randomization with a 1 to 4 allocation rate, actual group sizes of 72 in each low iron group and 36 in the CF group were chosen.
For statistical analyses, SPSS for Windows version 26.0 (SPSS Chicago, IL, USA) was used. The analyses concerning the lactoferrin intervention, and the secondary outcomes presented here, are all based on an assumption of superiority. Student t-test and analysis of variance (ANOVA) with Bonferroni adjusted post-hoc tests were used for group comparisons of continuous variables. Since we assumed not normally distributed data in the pre-study power calculations, cytokines were logarithmically transformed and group comparisons are presented as geometric means. For other not normally distributed continuous data, Mann-Whitney U test and Kruskal-Wallis rank-sum test were used. For comparison regarding categorical variables, Fisher exact test was used.
RESULTS
Study Groups
Recruitment was conducted between June 2014 and June 2018. Five infants dropped out before the first follow-up at 4 months and one additional between 4 and 6 months (Fig. 1). Background and baseline characteristics of the 247 infants assessed in the present study are presented in Table 1. There were no significant differences among the formula-fed groups or in comparison to the dropouts. Most (87%) of the formula-fed infants had received breastmilk during the first weeks of life for a median of 17 days, with no significant differences between randomization groups. Concerning compliance, three participants (1.7%) did not receive the study formula as main source of nutrition, whereof two (CF, Lf+) due to gastrointestinal symptoms, and one (Lf−) to extensive eczema. According to dietary records, all other infants were exclusively fed the study formula apart from small portions of complementary food (20).
TABLE 1.
Background and baseline characteristics in included participants
| Lf+ | Lf− | CF | BF | ||
| Background | n = 72 | n = 71 | n = 33 | P value | n = 71 |
| Female | 36 (50) | 36 (50.7) | 16 (48.5) | >0.999 | 36 (50.7) |
| Gestational age (wk) | 39.6 ± 1.2 | 39.8 ± 1.2 | 39.5 ± 1.3 | 0.339 | 39.8 ± 1.5 |
| Vaginal delivery | 52 (72.2) | 56 (78.9) | 26 (78.8) | 0.622 | 61 (85.9) |
| Apgar score at 5 min | 9.3 ± 0.8 | 9.3 ± 0.9 | 9.3 ± 0.9 | >0.999 | 9.0 ± 0.8 |
| Birth weight (kg) | 3.59 ± 0.44 | 3.54 ± 0.43 | 3.50 ± 0.37 | 0.535 | 3.54 ± 0.36 |
| Birth length (cm) | 50.1 ± 1.8 | 50.0 ± 1.8 | 50.3 ± 1.4 | 0.756 | 50.3 ± 1.9 |
| Head circumference (cm) | 35.2 ± 1.5 | 35.0 ± 1.3 | 34.9 ± 1.3 | 0.513 | 35.1 ± 1.0 |
| Neonatal unit care | 4 (5.6) | 5 (7.0) | 3 (9.1) | 0.739 | 2 (2.8) |
| Antibiotic treatment day 0–7 | 0 | 0 | 0 | 0 | |
| Maternal age (y) | 29.6 ± 4.1 | 29.9 ± 5.4 | 29.5 ± 4.3 | 0.146 | 30.8 ± 4.8 |
| Multiparous mother | 47 (65.3) | 42 (59.2) | 16 (48.5) | 0.269 | 38 (53.5) |
| Mother of non-Nordic descent | 1 (1.4) | 3 (4.2) | 1 (3.0) | 0.516 | 2 (2.8) |
| Maternal education at university level | 30 (41.7)∗ | 28 (39.4)∗ | 14 (42.4)∗ | 0.939 | 49 (69.0) |
| Maternal smoking during pregnancy | 4 (5.6) | 3 (4.2) | 0 | 0.528 | 0 |
| Iron supplementation during pregnancy | 55 (76.4) | 53 (74.6) | 27 (81.8) | 0.713 | 54 (76.1) |
Values are presented as mean ± SD or number (%).
P values for differences among intervention groups using analysis of variance for means, and Fisher exact test and Pearson chi-square for proportions.
BF = breastfed; CF = control formula; Lf− = no added lactoferrin; Lf+ = added lactoferrin; SD = standard deviation.
Significantly different from BF infants (P < 0.05).
The complete iron status at baseline and during intervention is presented elsewhere.
Cytokines
Blood samples for cytokine analyses were successfully collected from >94% of the infants remaining in the study at each assessment (Fig. 1). The results are presented in Table 2. Infants with CRP > 8 mg/L were excluded in all cytokine assessments. For IL-2, the values were generally low with 46% below the detection range (0.19 pg/mL) and hence extrapolated, whereof 14% set to zero. The prevalence of extrapolated values was evenly distributed between the study groups. No significant differences in cytokine levels were observed among the three study formula groups (Lf+, Lf−, and CF) at baseline or at 4, 6, or 12 months of age. Also the breastfed infants showed similar levels with two minor exceptions. When both low iron groups were pooled into one comparator group, TGF-β2 was significantly lower compared to the CF group at 6 months of age. No other significant differences were observed (Table 2, Supplemental Digital Content).
TABLE 2.
Geometric mean (95% CI) concentrations of cytokines at inclusion, 4, 6, and 12 mo in formula fed infants randomized to three different infant formulas and in a breastfed reference group
| Lf+ | Lf− | CF | P value | BF | |||||
| Inclusion | n = 68 | n = 67 | n = 36 | n = 70 | |||||
| TGF-β1 (ng/mL) | 59.6 | 56.1–63.4 | 59.5 | 56.5–62.7 | 63.1 | 58.1–68.5 | 0.436 | 58.2 | 55.4–61.1 |
| TGF-β2 (ng/mL) | 1.39 | 1.31–1.49 | 1.34 | 1.25–1.43 | 1.51∗ | 1.36–1.67 | 0.111 | 1.30 | 1.23–1.38 |
| TNF-α (pg/mL) | 16.9 | 15.4–18.6 | 15.9 | 14.5–17.4 | 16.6 | 15.0–18.4 | 0.615 | 16.6 | 15.4–17.9 |
| IL-2 (pg/mL) | 0.72∗ | 0.56–0.88 | 0.53 | 0.39–0.69 | 0.55 | 0.35–0.76 | 0.203 | 0.38 | 0.27–0.49 |
| 4 mo | n = 67 | n = 67–68 | n = 32 | n = 67 | |||||
| TGF-β1 (ng/mL) | 66.1 | 62.9–69.4 | 63.8 | 60.4–67.3 | 65.9 | 61.2–71.0 | 0.577 | 66.9 | 63.8–70.1 |
| TGF-β2 (ng/mL) | 1.67 | 1.58–1.77 | 1.61 | 1.50–1.72 | 1.73 | 1.57–1.91 | 0.378 | 1.65 | 1.55–1.75 |
| TNF-α (pg/mL) | 14.5 | 13.1–16.0 | 14.4 | 13.2–15.8 | 14.9 | 13.3–16.6 | 0.923 | 13.4 | 12.2–14.8 |
| IL-2 (pg/mL) | 0.93∗ | 0.70–1.17 | 1.03∗ | 0.78–1.30 | 0.95 | 0.54–1.42 | 0.862 | 0.56 | 0.40–0.74 |
| 6 mo | n = 67 | n = 67 | n = 31 | n = 66 | |||||
| TGF-β1 (ng/mL) | 60.7 | 57.4–64.2 | 58.4 | 55.1–61.7 | 62.3 | 58.3–66.5 | 0.347 | 57.4 | 54.5–60.5 |
| TGF-β2 (ng/mL) | 1.61 | 1.50–1.72 | 1.56 | 1.45–1.67 | 1.78 | 1.63–1.94 | 0.082 | 1.50 | 1.40–1.60 |
| TNF-α (pg/mL) | 16.2 | 15.1–17.4 | 15.9 | 14.9–17.0 | 16.0 | 14.3–17.8 | 0.924 | 16.9 | 15.8–18.1 |
| IL-2 (pg/mL) | 1.64 | 1.36–1.94 | 1.63 | 1.32–1.97 | 1.20 | 0.85–1.60 | 0.180 | 1.36 | 1.10–1.64 |
| 12 mo | n = 64 | n = 67 | n = 30 | n = 58 | |||||
| TGF-β1 (ng/mL) | 54.2 | 51.6–57.0 | 52.0 | 49.4–54.8 | 55.0 | 51.4–58.7 | 0.341 | 53.4 | 50.7–56.2 |
| TGF-β2 (ng/mL) | 1.51 | 1.41–1.61 | 1.45 | 1.35–1.56 | 1.60 | 1.47–1.75 | 0.267 | 1.52 | 1.42–1.62 |
| TNF-α (pg/mL) | 12.9 | 11.8–14.2 | 13.3 | 12.1–14.5 | 13.7 | 11.7–16.0 | 0.783 | 13.6 | 12.3–15.0 |
| IL-2 (pg/mL) | 1.93 | 1.61–2.27 | 1.99 | 1.67–2.32 | 1.64 | 1.26–2.06 | 0.428 | 1.84 | 1.57–2.12 |
Values are presented as geometric mean (95% CI).
P values for differences among intervention groups using analysis of variance for means.
BF = breastfed; CF = control formula; CI = confidence interval; IL = interleukin; Lf− = no added lactoferrin; Lf+ = added lactoferrin; TGF = transforming growth factor; TNF = tumor necrosis factor.
Significantly different from BF infants (P < 0.05) in an independent sample t-test.
Morbidity
Whereas there were several differences in reported morbidity between the study formula groups and the breastfed reference during the first 6 months, no significant differences were observed among study formula groups. Cumulative incidence and/or longitudinal prevalence of fever, cough, breathing difficulties, upper respiratory infections, gastroenteritis, otitis media, doctor visits, and infection related hospitalization were similar in all three groups (Table 3). A significantly higher proportion of infants in the Lf+ group were treated with antipyretics compared to the Lf− group but there was no difference compared to the CF group and no significant differences in longitudinal prevalence for the same outcome. There were no significant differences in treatment with antibiotics, in-hospital given inhalation medication (salbutamol, adrenaline, or saline) or in hospital stays.
TABLE 3.
Morbidity and use of infection-related medication between 6 weeks and 12 mo of age in formula fed infants (n = 176) randomized to three different infant formulas and in a breastfed reference group (n = 71)
| 6 weeks–6 mo | 6–12 mo | |||||||||
| Lf+ | Lf− | CF | P value | BF | Lf+ | Lf− | CF | P value | BF | |
| Cumulative incidence, n (%) | ||||||||||
| Fever | 46 (64.8) | 45 (64.8) | 19 (57.6) | 0.751 | 36 (50.7) | 55 (84.6) | 48 (77.4) | 20 (74.1) | 0.447 | 52 (80.0) |
| Upper respiratory infection | 64 (88.9) | 60 (84.5) | 27 (81.8) | 0.536 | 57 (81.4) | 66 (93.0) | 63 (90.0) | 27 (81.8) | 0.228 | 65 (94.2) |
| Cough | 48 (67.6) | 42 (59.2) | 20 (60.6) | 0.591 | 38 (53.5) | 49 (75.4) | 33 (53.2) | 23 (82.1) | 0.005 | 43 (66.2) |
| Breathing difficulties | 7 (9.9) | 12 (16.9) | 5 (15.2) | 0.500 | 5 (7.0) | 9 (13.8) | 9 (14.5) | 6 (22.2) | 0.586 | 7 (10.8) |
| Otitis (antibiotic treated) | 5 (6.9) | 3 (4.2) | 3 (9.1) | 0.510 | 1 (1.4) | 4 (5.6) | 8 (11.6) | 5 (15.2) | 0.234 | 2 (2.9) |
| Gastroenteritis | 10 (13.9)∗ | 9 (12.7)∗ | 3 (9.1) | 0.874 | 1 (1.4) | 12 (16.9) | 13 (18.8) | 8 (24.2) | 0.672 | 20 (29.0) |
| Physician visit | 21 (29.2) | 23 (31.9)∗ | 14 (38.9)∗ | 0.626 | 11 (15.3) | 31 (43.1) | 32 (44.4) | 24 (66.7)∗ | 0.053 | 21 (29.2) |
| Infection-related hospitalization | 5 (6.9) | 2 (2.8) | 3 (9.1)∗ | 0.362 | 0 (0) | 3 (4.2) | 3 (4.3) | 0 (0.0) | 0.654 | 1 (1.4) |
| Use of antipyretics | 61 (84.7)∗ | 48 (67.6)∗ | 23 (69.7) | 0.048 | 35 (50.0) | 64 (90.1) | 58 (84.1) | 27 (81.8) | 0.443 | 54 (78.3) |
| Systemic antibiotic treatment | 8 (11.1) | 4 (5.6) | 3 (9.1) | 0.528 | 2 (2.9) | 5 (7.0) | 9 (13.0) | 5 (15.2) | 0.352 | 3 (4.3) |
| In hospital use of inhaled drug/saline | 5 (7.1) | 6 (8.7) | 4 (12.5) | 0.641 | 3 (4.4) | 2 (2.8) | 1 (1.4) | 2 (6.1) | 0.418 | 3 (4.3) |
| Longitudinal prevalence, % (Q1;Q3) | ||||||||||
| Days with fever | 1.4 (0.0;2.9) | 0.8 (0.0;1.8) | 0.8 (0.0;2.7) | 0.768 | 0.7 (0.0;1.5) | 2.2 (1.1;3.7) | 1.9 (1.0;3.4) | 2.2 (1.6;5.4) | 0.635 | 2.4 (1.0;3.3) |
| Days with upper respiratory infection | 7.9 (2.9;13.8) | 5.6 (2.8;14.4) | 5.1 (1.8;9.7) | 0.459 | 7.8 (1.7;15.5) | 12.1 (6.6;19.8) | 8.2 (3.2;8.2) | 13.4 (5.9;22.1) | 0.089 | 10.8 (6.4;17.5) |
| Days with cough | 3.2 (0.0;8.8) | 4.4 (0.0;10.1) | 3.3 (0.0;7.0) | 0.751 | 1.6 (0.0;7.4) | 3.8 (0.3;12.6) | 1.8 (0.0;11.2) | 9.1 (2.7;16.4) | 0.075 | 3.0 (0.0;9.9) |
| Days with breathing difficulties | 0.0 (0.0;0.0) | 0.0 (0.0;0.0) | 0.0 (0.0;0.0) | 0.529 | 0.0 (0.0;0.0) | 0.0 (0.0;0.0) | 0.0 (0.0;0.0) | 0.0 (0.0;0.6) | 0.387 | 0.0 (0.0;0.0) |
| Days with gastroenteritis | 0.0 (0.0;0.0)∗ | 0.0 (0.0;0.0) | 0.0 (0.0;0.0) | 0.251 | 0.0 (0.0;0.0) | 0.0 (0.0;0.0) | 0.0 (0.0;0.0) | 0.0 (0.0;0.6) | 0.550 | 0.0 (0.0;0.6) |
| Days with stomach ache | 0.7 (0.0;3.0) | 0.0 (0.0;2.4) | 0.0 (0.0;4.4) | 0.543 | 0.0 (0.0;0.8) | 0.0 (0.0;0.5) | 0.0 (0.0;0.0) | 0.0 (0.0;1.9) | 0.132 | 0.0 (0.0;0.0) |
| Days with sleeping disturbance | 1.9 (0.0;7.7) | 2.0 (0.0;7.3) | 1.1 (0.0;11.1) | 0.966 | 1.2 (0.0;6.3) | 4.7 (1.1;15.2) | 3.5 (0.0;14.9) | 5.0 (2.3;11.1) | 0.761 | 2.6 (0.0;9.3) |
| Days with rash/eczema | 0.0 (0.0;0.0) | 0.0 (0.0;0.0) | 0.0 (0.0;0.8) | 0.666 | 0.0 (0.0;0.8) | 0.0 (0.0;2.0) | 0.3 (0.0;3.5) | 0.0 (0.0;3.8) | 0.247 | 0.0 (0.0;0.8) |
| Days with antipyretics | 1.5 (0.0;3.0)∗ | 1.2 (0.0;2.3)∗ | 1.2 (0.0;2.8)∗ | 0.660 | 0.0 (0.0;1.6) | 2.7 (0.6;5.2) | 1.7 (0.0;4.2) | 3.2 (0.9;5.7) | 0.326 | 1.5 (0.0;3.3) |
| Days with systemic antibiotics | 0.0 (0.0;0.0) | 0.0 (0.0;0.0) | 0.0 (0.0;0.0) | 0.368 | 0.0 (0.0;0.0) | 0.0 (0.0;0.0) | 0.0 (0.0;0.0) | 0.0 (0.0;0.7) | 0.264 | 0.0 (0.0;0.0) |
Values are presented as cumulative incidence, that is, number (%) of infants with each outcome or longitudinal prevalence, that is, median percentage (25th;75th percentile) of days with each outcome.
P values for differences among intervention groups using Kruskal-Wallis one-way test, Chi-square test or Fisher exact test.
BF = breastfed; CF = control formula; Lf− = no added lactoferrin; Lf+ = added lactoferrin; URI = upper respiratory infection.
Significantly different from BF infants (P < 0.05).
Between 6 and 12 months of life there were no significant differences among the study formula groups concerning cumulative incidence or longitudinal prevalence of fever, breathing difficulties, upper respiratory infections, gastroenteritis, or otitis media. Both the Lf+ and CF groups reported significantly higher incidence of cough than the Lf− group.
From 6 to 12 months, a numerically higher incidence of doctor visits was demonstrated for the CF group compared to each of the low iron formula groups. No differences were found between the two low iron formula groups. No significant differences were observed concerning infection related hospital stays, use of antipyretics or antibiotics. There were no significant differences in reported incidence of in-hospital inhalation treatment, but the CF infants showed a significantly higher longitudinal prevalence compared to infants in both low iron formula groups.
DISCUSSION
In this study, we hypothesized that adding bovine lactoferrin and lowering the iron content in infant formula would affect inflammatory response and secondary, affect morbidity in healthy normal birth weight infants. Our hypotheses were not supported. We did not reveal any apparent effect on cytokine concentrations at 4, 6, or 12 months from adding bovine lactoferrin, and only minor effects on two cytokines at 6 months of age after receiving a low iron formula. Apart from a higher incidence of antipyretic treatment among infants receiving formula with added lactoferrin, no significant differences in morbidity were observed in any of the study formula groups during the first 6 months and only a few and diverging effects were observed between 6 and 12 months.
One of the most well-documented benefits of breastfeeding compared to feeding formula is the difference observed in inflammatory response and risk of infections. Infants receiving human milk for longer periods have lower infectious morbidity and mortality, due to an assumed protective effect against diarrhea, respiratory infections and, in infants younger than two years, also against otitis media (1). With regard to reliable biomarkers, it is well documented that breastfed infants have a more beneficial gut microbiome, but also different maturation of the inflammatory response compared to formula-fed infants (3,21–23). Kainonen et al (21) compared 18 formula-fed infants with 29 breastfed peers and in the latter found a persistent anti-inflammatory response with higher concentration of the anti-inflammatory cytokine TGF-β2 and lower concentrations of the proinflammatory cytokines IL-2 and TNF-α during the first year of life. They concluded that breastfeeding seems to promote an anti-inflammatory cytokine milieu with possible positive consequences for development and regulation of the immune system and tolerance and suggested that in the efforts of improve infant formula, research should aim at achieving similar physiological effects in formula-fed infants.
In the present study, we were not able to show a more beneficial cytokine profile following any of the two interventions tested. In fact, the only significant finding was, at 6 months of age, lower levels of TGF-β2 in the infants fed low iron formula compared to the CF group (Table 2, Supplemental Digital Content). Since TGF-β2 is considered an anti-inflammatory cytokine, this finding is in opposite to our hypothesis. A mechanistic explanation to this unexpected and isolated finding is not present and the result may represent a type I error. It should be noted that in contrast to Kainonen et al (24), this study demonstrated no clear differences between breastfed and formula-fed infants, including the CF group. Consequently, there was no cytokine profile to improve with the two interventions. This may reflect a shift towards a more breastfed-like physiological response in all formula groups, including the CF group, possibly attributed to other components in the formulas. The explanation may also be found in a too small study population, or inadequate methods for measuring cytokines. Particularly for IL-2, the ELISA used was not sensitive enough for the lower levels. We found no other infant formula studies exploring the effects of lactoferrin, or iron, on cytokine concentrations to compare our results; however, cytokines as a biomarker of immune response in infant formula studies have previously been used successfully.
To observe effects on clinically relevant symptoms of disease, we used a validated local tool (25) to assess morbidity throughout the intervention and the following 6 months. Interestingly considering the low-risk environment, several of the morbidity outcomes, that is, gastroenteritis, physician visits and use of antipyretics, were all less prevalent in the breastfed reference group, supporting the main assumption that breastfed infants are protected from morbidity; however, similar to the analyses of cytokines, few or no significant effects of the interventions were observed. Three previous formula studies have reported a positive effect of lactoferrin on incidence of infections in normal birth weight infants or toddlers (6,7,9). King et al (6) enrolled 54 normal birth weight infants <4 weeks of age to receive formula at 850 mg bovine lactoferrin/L (n = 26) versus 102 mg bovine lactoferrin/L (controls, n = 26) for 12 months. They showed a reduced rate of low respiratory illness (0.15/year vs 0.5/year) but no effect on otitis, URI, colic, diarrhea, or duration of illness. Chen et al (7) randomized 260 Chinese previously exclusively breastfed infants, between 4 and 6 months to receive a lactoferrin-fortified formula (lactoferrin 38 mg/100 g, n = 130) or a formula without added lactoferrin (n = 130) for 3 months. They found a significantly lower incidence of respiratory illness and diarrhea following lactoferrin supplementation. Finally, a recent Japanese study reported significantly lower total days of respiratory symptoms and prevalence of acute gastrointestinal symptoms for toddlers, 12–32 months old, who received lactoferrin supplemented formula for 13 weeks (9).
In parallel with the present and previous studies exploring the effects of lactoferrin as infant formula component, an important research field concerning neonates and lactoferrin must also be mentioned for an overall picture. In 2009, Manzoni et al (8) found a significant reduction in the incidence of late-onset sepsis in very low birth weight neonates receiving supplementation with bovine lactoferrin. Since then, many well-powered randomized trials have been launched with the aims to confirm the results (26–28). Several metanalyses and Cochrane reviews have focused on the field (12,27). In 2020, Pammi and Gautham (12) summarized results from 12 previous studies including in total 5425 preterm infants. With a low certainty of evidence, they found a decrease in blood infection, including fungal infection, and a decrease in gastrointestinal injury when giving lactoferrin in combination with probiotics. Despite wide efforts in preterm studies, questions remain regarding the choice of lactoferrin, preparations, and doses, all highlighting the complexity and need of repeated studies.
In the present study, we did not reveal any significant effect on cytokines or morbidity from lowering the concentration of iron fortification. We have previously presented that there were no significant differences in iron status or growth among the formula groups at 4 and 6 months except for a small difference in ferritin at 6 months (20). The importance of iron for the health of infants and children is well-documented (14,17). Among many effects iron is essential for immune function, but also a necessary nutrient for almost all microorganisms (29). An increase in colonic iron, however, may shift the gut microbiota towards abundance of pathogens at the expense of beneficial bacteria strains like bifidobacteria and lactobacilli, with a subsequent increase in inflammation and possibly diarrhea (30,31). Therefore, exploring effects on immune function is important when determining the optimal level of iron fortification. Observations concerning the effect of iron supplement on gastrointestinal symptoms, infections and other morbidity are diverging and few studies have assessed the effects of different levels of iron fortification (19,32–34).
Our study is one of the largest studies on lactoferrin fortification in healthy term infants and the first to explore the impact of low iron formula on cytokines and morbidity. The results are reliable since the study was well powered concerning primary outcomes, used a randomized design, had low drop-out rates and a high degree of compliance. The most likely conclusion is therefor that the interventions did not significantly affect the outcomes studied in the present setting.
Limitations in study design include the absence of a fourth group receiving high iron formula with lactoferrin (2 × 2 design) and the lack of power concerning parts of the secondary outcomes. A 2 × 2 study design would reveal a possible interactive effect of iron and lactoferrin. The decisions regarding number of study arms and group sizes were based on time and financial considerations. Another important factor when interpreting the results is the choice of lactoferrin preparation. Previous studies on lactoferrin supplementation of term and preterm infants have used different commercial sources of lactoferrin and it has been shown that these exhibit highly varying biological activities in vitro (35), which may have contributed to the different outcomes observed among studies. It should also be underscored that for several of the outcomes studied here, including the main outcome (cytokines), there were no or few differences between the breastfed reference and the CF group, representing standard formula. Consequently, in the present setting, those outcomes were not optimal to demonstrate a reduced gap between formula-fed and breastfed infants.
Finally, when interpreting the results, the privileged socioeconomic context and homogenous study population must be taken into consideration. Most of the previous lactoferrin studies in term infants and children were conducted in lower socioeconomic contexts with higher burden of infections and under-five mortality rates compared to our study population. An improvement of infant formula supporting immunological development and health would probably leave greater imprint in the most vulnerable areas; however, not to forget, the greatest impact on the health and development of infants and children, will come from efforts to promote breastfeeding.
CONCLUSIONS
The present study did not to show any evident or lasting effect on inflammatory response or morbidity when lowering iron content or adding bovine lactoferrin to infant formula given to healthy term infants. Nevertheless, it confirms, in agreement with earlier observations, that both adjustments are safe with no observed adverse effects seen in the outcomes included. Evaluations of vaccine response and neurodevelopment will be performed to further examine potential benefits and risks. It should be noted that the study was set up in a privileged socioeconomic context with a low burden of infectious diseases and further studies are welcomed in more vulnerable populations.
Supplementary Material
Supplementary Material
Acknowledgments
We thank our research nurses Carina Forslund, Marie Lindvall and Maine Forsberg for dedicated field work, and data collection. We also thank Carina Lagerqvist, Mona Svensson and Eva-Lotta Andersson for professional help with laboratory analyses.
Footnotes
Mead Johnson Nutrition funded the study and provided the infant formula. This work was also supported by grants from Knut and Alice Wallenberg foundation, by regional agreement between Umeå University and Västerbotten County Council (ALF), the Oskar foundation and the KEMPE foundation. The funders had no role in study design, data collection, analyses, decision to publish, or preparation of the manuscript. Bo Lönnerdal and Olle Hernell have received honorarium for lectures and consultancy from Mead Johnson Nutrition.
The authors report no other conflicts of interest.
The study was registered at clinicaltrials.gov NCT02103205.
Supplemental digital content is available for this article.
REFERENCES
- 1.Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016; 387:475–490. [DOI] [PubMed] [Google Scholar]
- 2.Demmelmair H, Prell C, Timby N, et al. Benefits of lactoferrin, osteopontin and milk fat globule membranes for infants. Nutrients 2017; 9:817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr LE, Virmani MD, Rosa F, et al. Role of human milk bioactives on infants’ gut and immune health. Front Immunol 2021; 12: 604080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legrand D. Overview of lactoferrin as a natural immune modulator. J Pediatr 2016; 173: (Suppl): S10–S15. [DOI] [PubMed] [Google Scholar]
- 5.Sienkiewicz M, Jaskiewicz A, Tarasiuk A, et al. Lactoferrin: an overview of its main functions, immunomodulatory and antimicrobial role, and clinical significance. Crit Rev Food Sci Nutr 2021; 8:1–18. [DOI] [PubMed] [Google Scholar]
- 6.King JC, Jr, Cummings GE, Guo N, et al. A double-blind, placebo-controlled, pilot study of bovine lactoferrin supplementation in bottle-fed infants. J Pediatr Gastroenterol Nutr 2007; 44:245–251. [DOI] [PubMed] [Google Scholar]
- 7.Chen K, Chai L, Li H, et al. Effect of bovine lactoferrin from iron-fortified formulas on diarrhea and respiratory tract infections of weaned infants in a randomized controlled trial. Nutrition 2016; 32:222–227. [DOI] [PubMed] [Google Scholar]
- 8.Manzoni P, Rinaldi M, Cattani S, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA 2009; 302:1421–1428. [DOI] [PubMed] [Google Scholar]
- 9.Motoki N, Mizuki M, Tsukahara T, et al. Effects of lactoferrin-fortified formula on acute gastrointestinal symptoms in children aged 12–32 months: a randomized, double-blind, placebo-controlled trial. Front Pediatr 2020; 8:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochoa TJ, Pezo A, Cruz K, et al. Clinical studies of lactoferrin in children. Biochem Cell Biol 2012; 90:457–467. [DOI] [PubMed] [Google Scholar]
- 11.Group ETI. Enteral lactoferrin supplementation for very preterm infants: a randomised placebo-controlled trial. Lancet 2019; 393:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pammi M, Suresh G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2020; 3:CD007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lonnerdal B. Bioactive proteins in human milk: health, nutrition, and implications for infant formulas. J Pediatr 2016; 173: (Suppl): S4–S9. [DOI] [PubMed] [Google Scholar]
- 14.Domellof M, Braegger C, Campoy C, et al. Iron requirements of infants and toddlers. J Pediatr Gastroenterol Nutr 2014; 58:119–129. [DOI] [PubMed] [Google Scholar]
- 15.Baker RD, Greer FR. Committee on Nutrition American Academy of Diagnosis and Prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). Pediatrics 2010; 126:1040–1050. [DOI] [PubMed] [Google Scholar]
- 16.Lonnerdal B. Excess iron intake as a factor in growth, infections, and development of infants and young children. Am J Clin Nutr 2017; 106:1681s–1687s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georgieff MK, Krebs NF, Cusick SE. The benefits and risks of iron supplementation in pregnancy and childhood. Annu Rev Nutr 2019; 39:121–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agrawal S, Berggren KL, Marks E, et al. Impact of high iron intake on cognition and neurodegeneration in humans and in animal models: a systematic review. Nutr Rev 2017; 75:456–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasricha SR, Hayes E, Kalumba K, et al. Effect of daily iron supplementation on health in children aged 4–23 months: a systematic review and meta-analysis of randomised controlled trials. Lancet Glob Health 2013; 1:e77–e86. [DOI] [PubMed] [Google Scholar]
- 20.Bjormsjo M, Hernell O, Lonnerdal B, et al. Reducing iron content in infant formula from 8 to 2 mg/L does not increase the risk of iron deficiency at 4 or 6 months of age: a randomized controlled trial. Nutrients 2020; 13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kainonen E, Rautava S, Isolauri E. Immunological programming by breast milk creates an anti-inflammatory cytokine milieu in breast-fed infants compared to formula-fed infants. Br J Nutr 2013; 109:1962–1970. [DOI] [PubMed] [Google Scholar]
- 22.Ho NT, Li F, Lee-Sarwar KA, et al. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat Commun 2018; 9:4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Backhed F, Roswall J, Peng Y, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015; 17:690–703. [DOI] [PubMed] [Google Scholar]
- 24.Lonnerdal B, Kvistgaard AS, Peerson JM, et al. Growth, nutrition, and cytokine response of breast-fed infants and infants fed formula with added bovine osteopontin. J Pediatr Gastroenterol Nutr 2016; 62:650–657. [DOI] [PubMed] [Google Scholar]
- 25.Timby N, Hernell O, Vaarala O, et al. Infections in infants fed formula supplemented with bovine milk fat globule membranes. J Pediatr Gastroenterol Nutr 2015; 60:384–389. [DOI] [PubMed] [Google Scholar]
- 26.Ochoa TJ, Zegarra J, Bellomo S, et al. Randomized controlled trial of bovine lactoferrin for prevention of sepsis and neurodevelopment impairment in infants weighing less than 2000 grams. J Pediatr 2020; 219:118.e5–125.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarnow-Mordi WO, Abdel-Latif ME, Martin A, et al. The effect of lactoferrin supplementation on death or major morbidity in very low birthweight infants (LIFT): a multicentre, double-blind, randomised controlled trial. Lancet Child Adolesc Health 2020; 4:444–454. [DOI] [PubMed] [Google Scholar]
- 28.Enteral lactoferrin supplementation for very preterm infants: a randomised placebo-controlled trial. Lancet 2019; 393:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kortman GA, Raffatellu M, Swinkels DW, et al. Nutritional iron turned inside out: intestinal stress from a gut microbial perspective. FEMS Microbiol Rev 2014; 38:1202–1234. [DOI] [PubMed] [Google Scholar]
- 30.Paganini D, Zimmermann MB. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: a review. Am J Clin Nutr 2017; 106:1688s–1693s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simonyte Sjodin K, Domellof M, Lagerqvist C, et al. Administration of ferrous sulfate drops has significant effects on the gut microbiota of iron-sufficient infants: a randomised controlled study. Gut 2019; 68:2095–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dewey KG, Domellof M, Cohen RJ, et al. Iron supplementation affects growth and morbidity of breast-fed infants: results of a randomized trial in Sweden and Honduras. J Nutr 2002; 132:3249–3255. [DOI] [PubMed] [Google Scholar]
- 33.Berger J, Ninh NX, Khan NC, et al. Efficacy of combined iron and zinc supplementation on micronutrient status and growth in Vietnamese infants. Eur J Clin Nutr 2006; 60:443–454. [DOI] [PubMed] [Google Scholar]
- 34.Lind T, Seswandhana R, Persson LA, et al. Iron supplementation of iron-replete Indonesian infants is associated with reduced weight-for-age. Acta Paediatr 2008; 97:770–775. [DOI] [PubMed] [Google Scholar]
- 35.Lonnerdal B, Du X, Jiang R. Biological activities of commercial bovine lactoferrin sources. Biochem Cell Biol 2021; 99:35–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.