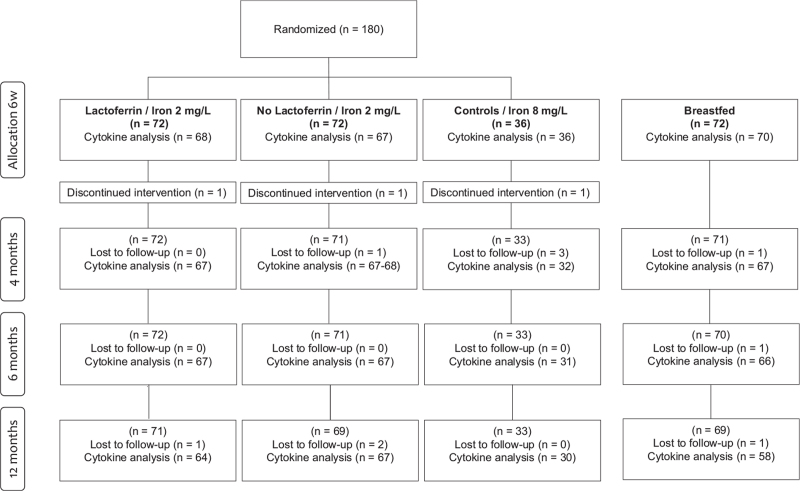
FIGURE 1.
Trial profile. Three infants discontinued intervention due to gastrointestinal symptoms (n = 2, CF, LF+) and extensive eczema (n = 1, LF−). They remained in the study. Six infants were lost to follow-up due to early withdrawal (n = 2, Lf−, BF), gastrointestinal side effects (n = 3, CF) and extensive data collection (n = 1, BF). Additional four infants were lost to follow up at 12 months of age (reasons not given). BF = breastfed; CF = control formula; Lf− = no added lactoferrin; Lf+ = added lactoferrin.