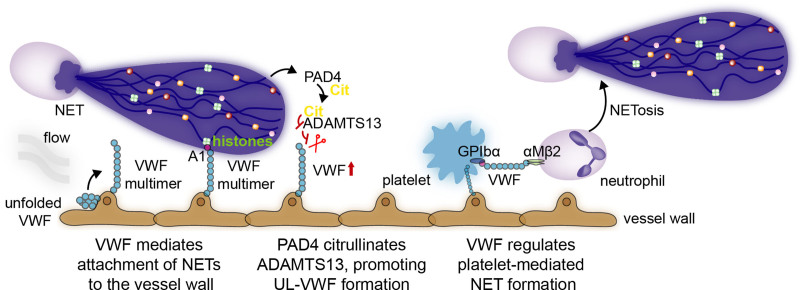
Figure 3.
VWF (Von Willebrand factor) and neutrophil extracellular traps (NETs) interact via various mechanisms. In response to shear stress of flowing blood, ultra-large VWF multimers elongate and expose the A1 domain. Via different mechanisms, NETs and neutrophils interact with these ultra-large VWF multimers. First, the A1 domain on VWF binds NET histones and DNA, thus allowing them to stay in place and damage the vasculature. Second, by releasing PAD4, NETs can promote ADAMTS13 (A disintegrin and metalloprotease with a thrombospondin type 1 motif, member 13) citrullination, a protease that cleaves VWF, and subsequently abolish its activity, promoting ultra-large VWF-platelet string formation. VWF, in turn, can regulate platelet-induced NET formation by acting on platelet GPIbα and neutrophil αMβ2.