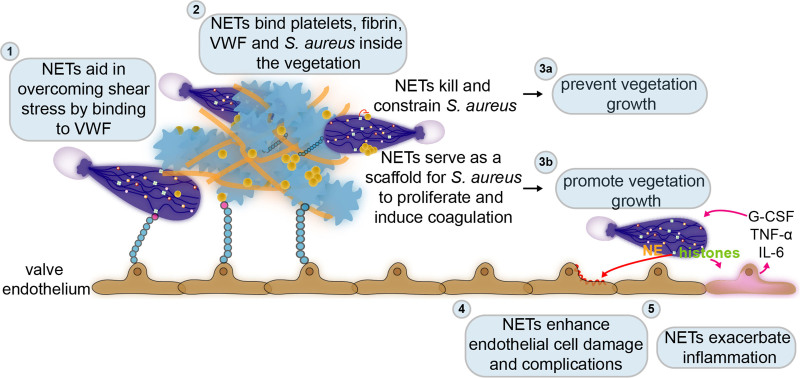
Figure 6.
The dual role of neutrophil extracellular traps (NETs) in infective endocarditis. Few diseases highlight the crucial interplay between coagulation, bacteria, and immunity better than infective endocarditis, which at its core is an infected blood clot attached to the cardiac valves. (1) Already at the very early stages of this disease, NETs can overcome the turbulent flow of the valves’ environment by binding to the endothelium via VWF (von Willebrand factor). While attached to the endothelium, NETs provide a scaffold for binding of platelets, fibrin, and bacteria. (2) In the already formed vegetation or infected blood clot, NETs also bind platelets, fibrin, and S. aureus. (3) Inside the vegetation, NETs could either prevent its progression by killing and constraining S. aureus (3a) or enhance its formation by further stimulating coagulation or proliferation of S. aureus (3b). The infected blood clot or vegetation typically develops on damaged or inflamed cardiac valves. NETs may also induce further damage (4) and inflammation (5) in the valve endothelium. However, the exact role of NETs in infective endocarditis remains to be established. G-CSF indicates granulocyte colony-stimulating factor; IL-6, interleukin 6; and TNFα, tumor necrosis factor α.